Публикации Лаборатории Химической Трансформации Антибиотиков
-
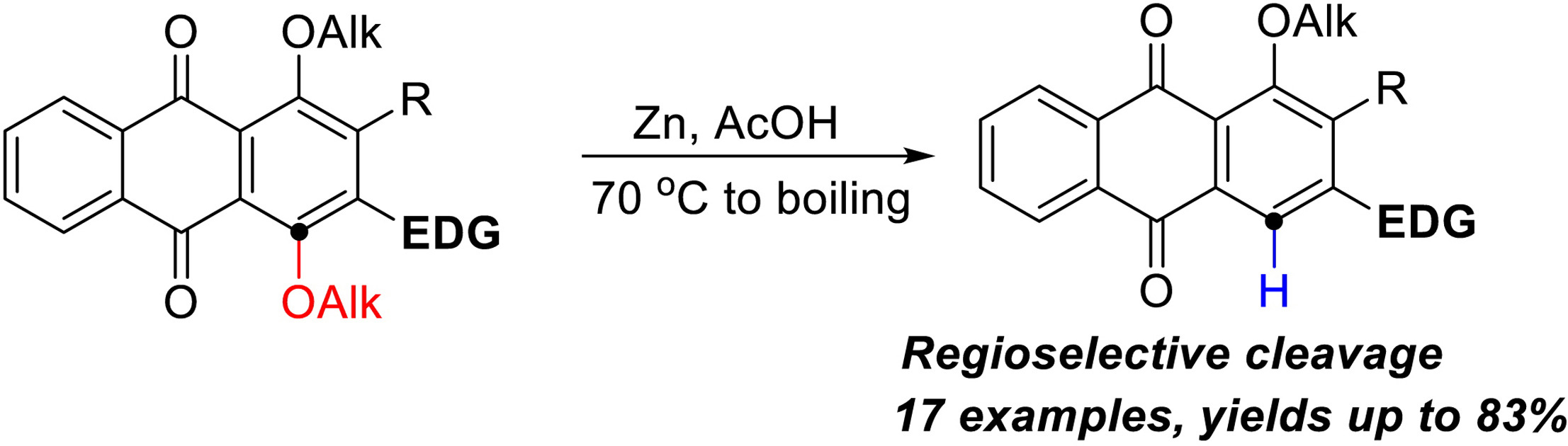
A.S. Tikhomirov, D.V. Andreeva, A.E. Shchekotikhin. Reductive elimination of alkoxy group in anthraquinone derivatives, Tetrahedron 2022, 122, 132957, https://doi.org/10.1016/j.tet.2022.132957.
-
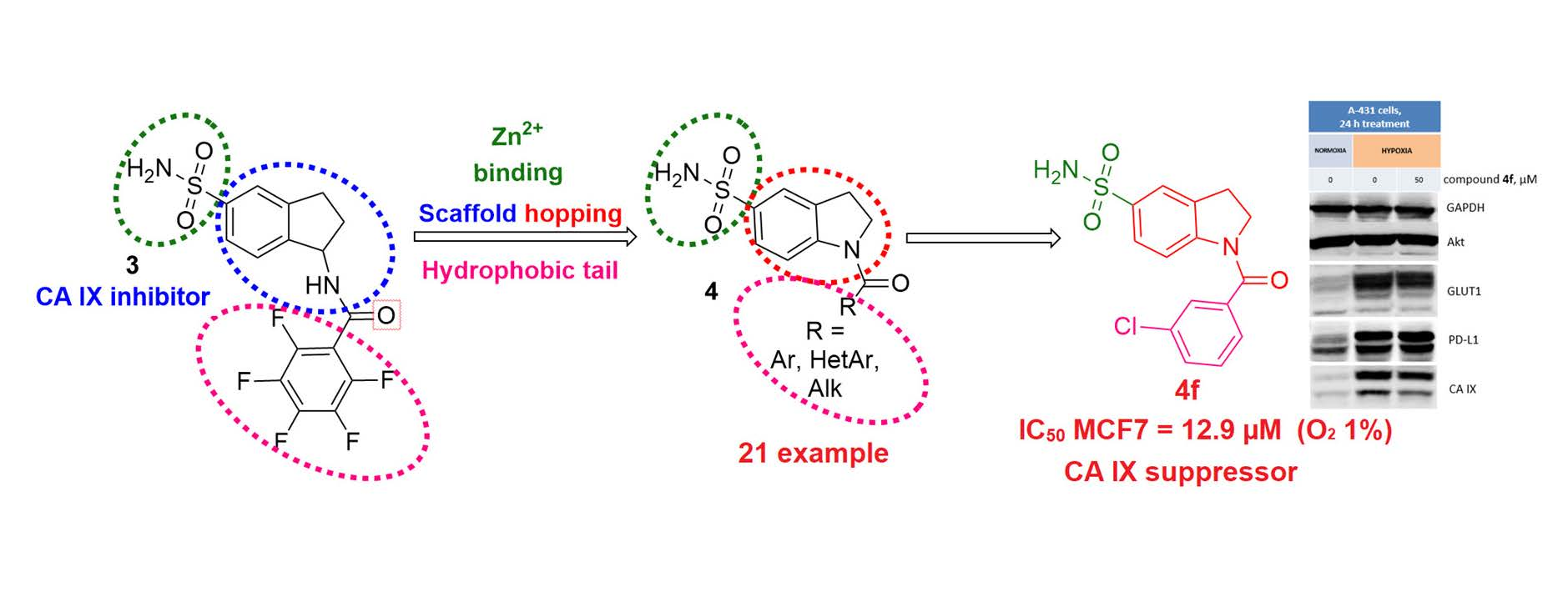
Krymov S. K., Scherbakov A.M., Dezhenkova L.G., Salnikova D.I., Solov’eva S.E., Sorokin D.V., Vullo D., De Luca V., Capasso C., Supuran C.T., Shchekotikhin A.E. Indoline-5-Sulfonamides: A Role of the Core in Inhibition of Cancer-Related Carbonic Anhydrases, Antiproliferative Activity and Circumventing of Multidrug Resistance. Pharmaceuticals, 2022, 15, 1453. https://doi.org/10.3390/ph15121453
-
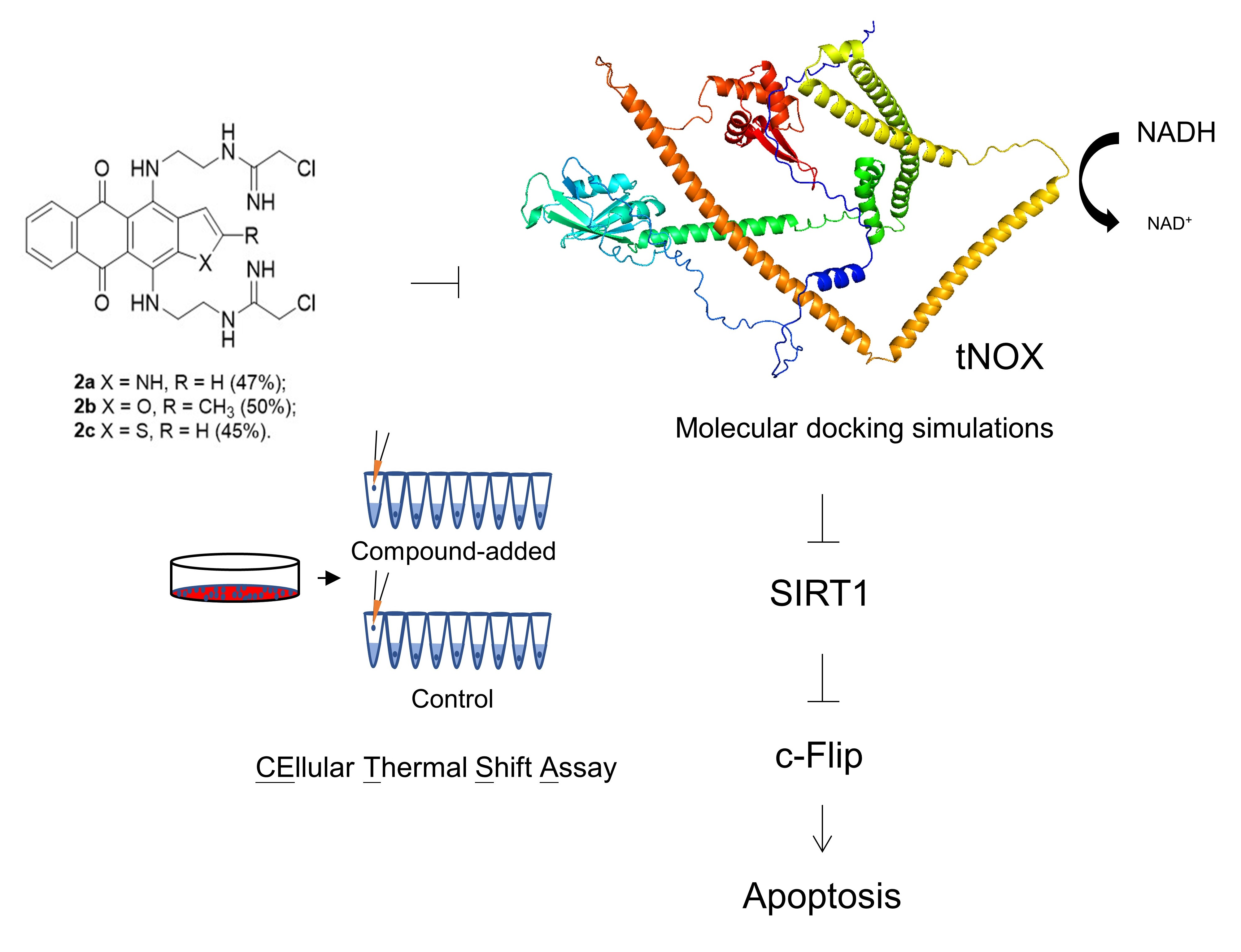
J.S. Chang, C.-Y. Chen, A.S. Tikhomirov, A. Islam,R.-H. Liang, C.-W. Weng, W.-H. Wu, A.E. Shchekotikhin, P.J. Chueh. Bis(chloroacetamidino)-Derived Heteroarene-Fused Anthraquinones Bind to and Cause Proteasomal Degradation of tNOX, Leading to c-Flip Downregulation and Apoptosis in Oral Cancer Cells. Cancers 2022, 14(19), 4719; https://doi.org/10.3390/cancers14194719
-
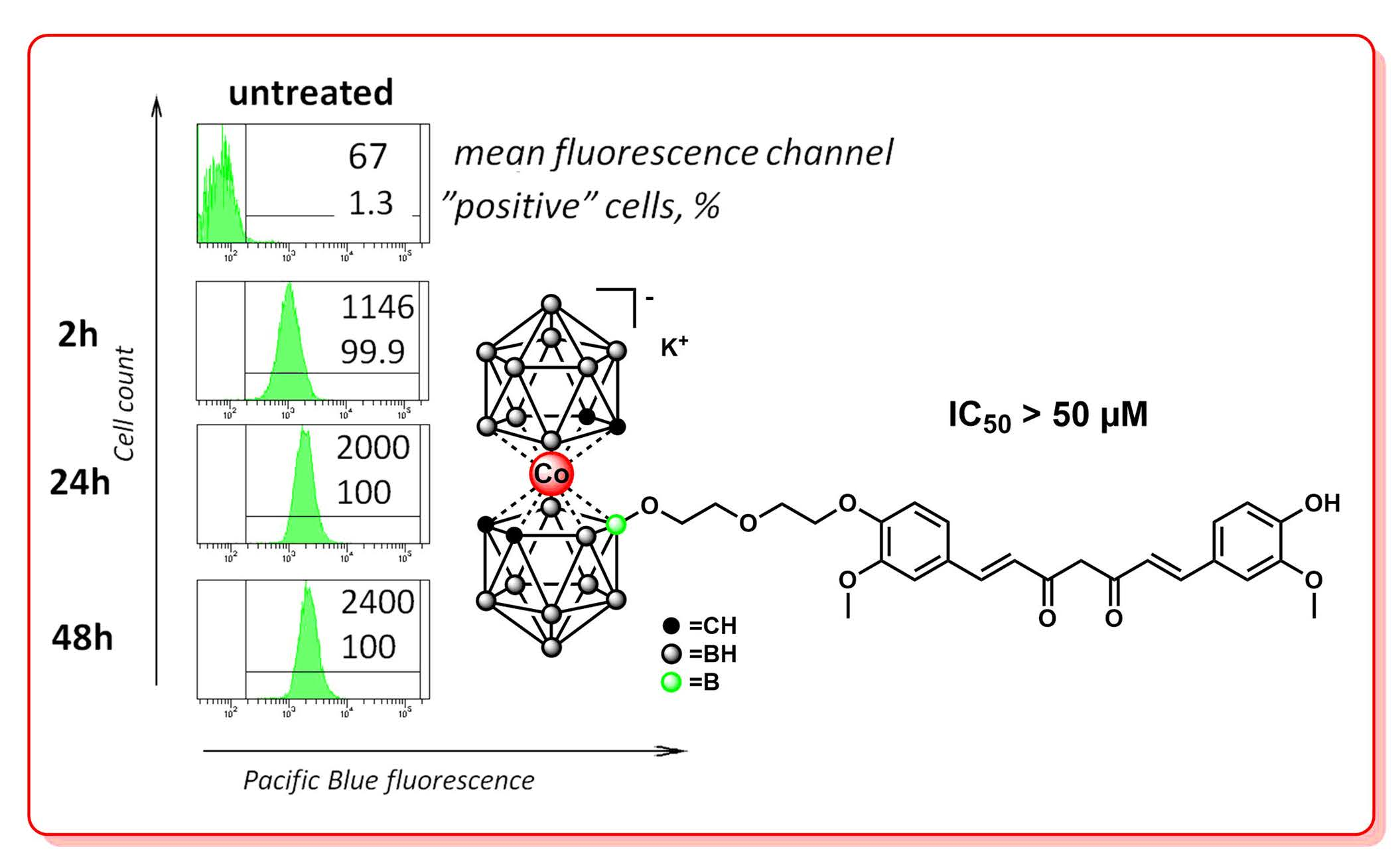
Dezhenkova L.G.; Druzina A.A.; Volodina Y.L.; Dudarova N.V.; Nekrasova N.A.; Zhidkova O.B.; Grin M.A.; Bregadze V.I. Synthesis of Cobalt Bis(Dicarbollide)-Curcumin Conjugates for Potential Use in Boron Neutron Capture Therapy. Molecules 2022, 27, 4658. DOI:10.3390/molecules27144658
-
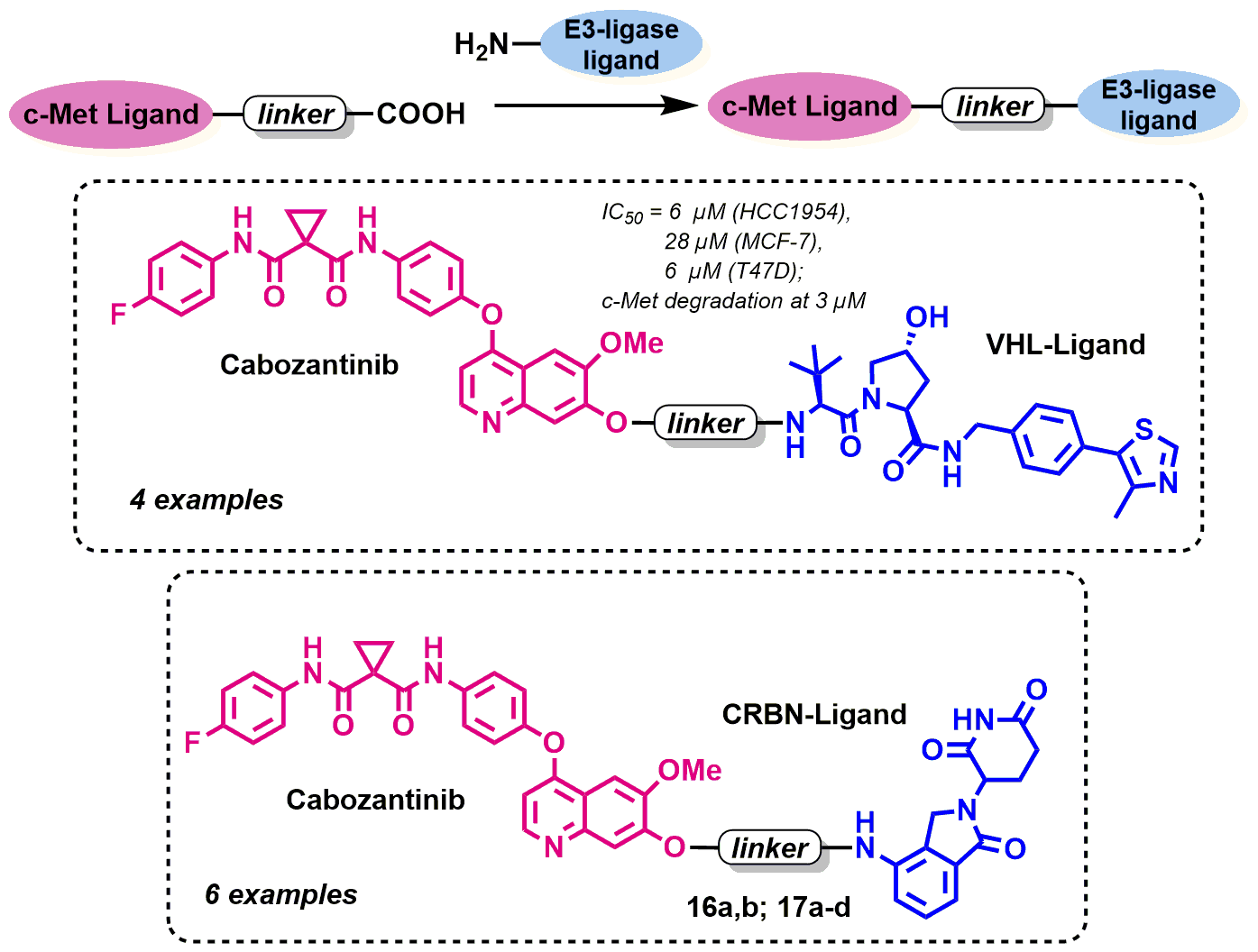
Sachkova, A.A.; Andreeva, D.V.; Tikhomirov, A.S.; Scherbakov, A.M.; Salnikova, D.I.; Sorokin, D.V.; Bogdanov, F.B.; Rysina, Y.D.; Shchekotikhin, A.E.; Shchegravina, E.S.;. Fedorov A. Yu Design, Synthesis and In Vitro Investigation of Cabozantinib-Based PROTACs to Target c-Met Kinase. Pharmaceutics 2022, 14, 2829. https://doi.org/10.3390/pharmaceutics14122829
-
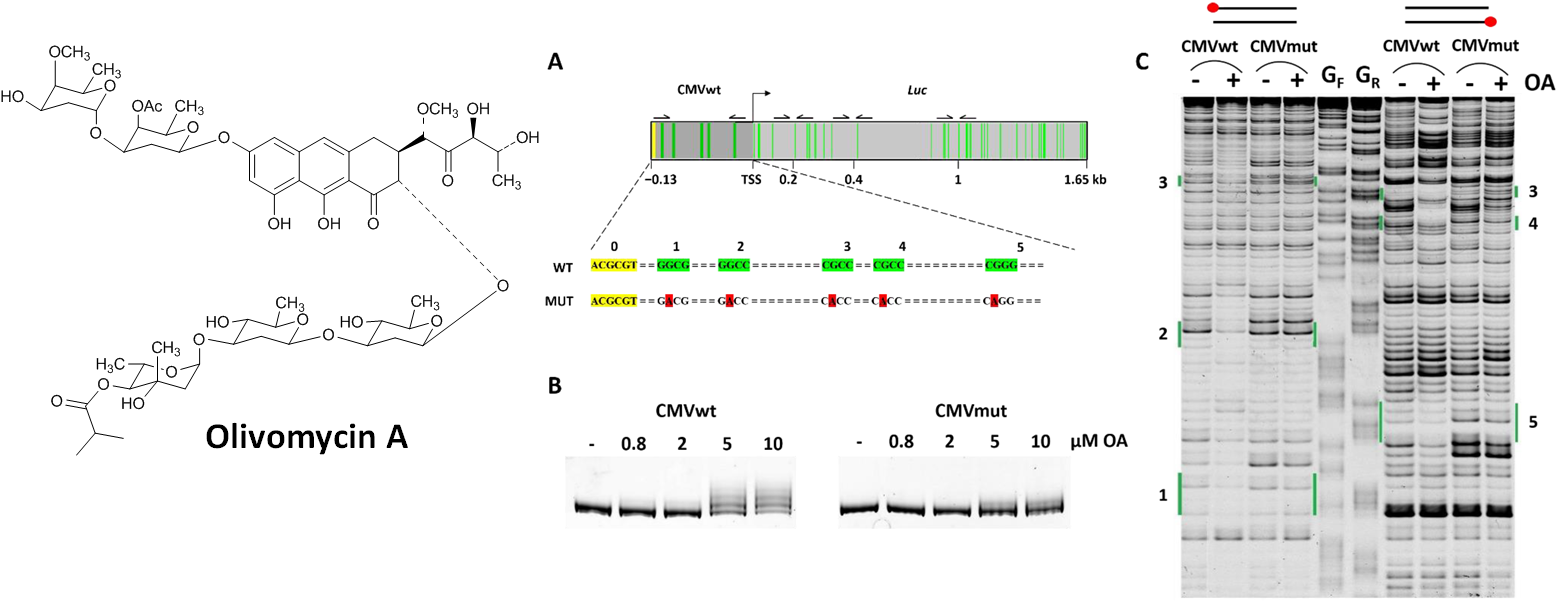
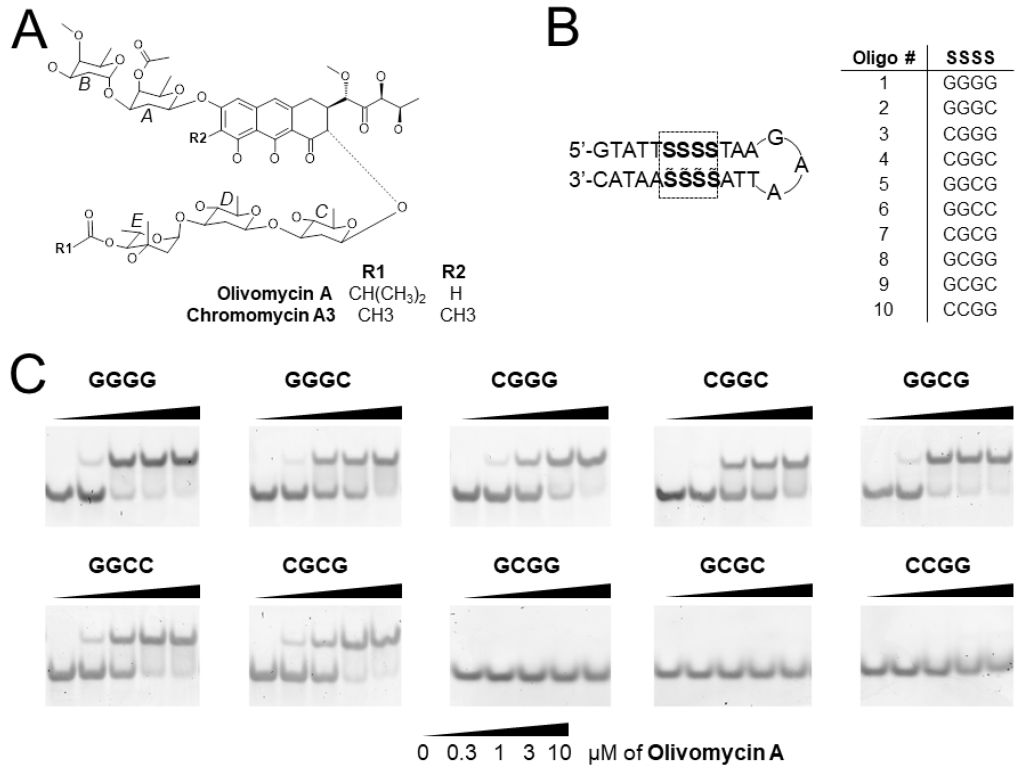
Isagulieva, A.K.; Kaluzhny, D.N.; Beniaminov, A.D.; Soshnikova, N.V.; Shtil, A.A. Differential Impact of Random GC Tetrad Binding and Chromatin Events on Transcriptional Inhibition by Olivomycin A. International Journal of Molecular Sciences 2022, 23, 8871. https://doi.org/10.3390/ijms23168871
-
M. Singh, Md.A. Haque, A.S. Tikhomirov, A.E. Shchekotikhin, U. Das, P. Kaur, Computational and Biophysical Characterization of Heterocyclic Derivatives of Anthraquinone against Human Aurora Kinase A. ACS Omega, 2022, 7, 44, 39603-39618, https://doi.org/10.1021/acsomega.2c00740
-
Исагулиева А.К., Тевяшова А.Н., Штиль А.А. Антибиотики группы ауреоловой кислоты: перспективы биологически активного класса соединений. Биоорганическая химия., 2022, 48(3), 1-13. doi:10.31857/S0132342322020129
-
N. Savin, A. Erofeev, V. Kolmogorov, S. Salikhov, Y. Efremov, P. Timashev, N. Grammatikova, I. Levshin, C. Edwards, Y. Korchev, P. Gorelkin. Scanning ion-conductance microscopy technique for studying the topography and mechanical properties of Candida parapsilosis yeast microorganisms; Biomaterials Science 2022. DOI:10.1039/d2bm00964a
-
Олсуфьева Е.Н., Янковская В.С., Дунченко Н.И. Обзор рисков контаминации антибиотиками молочной продукции. Антибиотики и химиотерапия 2022, 67 (7–8):82-96. https://doi.org/10.37489/0235-2990-2022-67-7-8-82-96
-
Щекотихин А. Е., Олсуфьева Е. Н., Янковская В. С. Антибиотики и родственные вещества. М.: Лаборатория знаний. 2022., 511 с. ISBN 978-5-93208-247-8.
-
A. Shchekotikhin, V.F Traven, N.A. Pozharskaya. History and Scientific Achievements of the Department of Organic Chemistry of the Mendeleev University of Chemical Technology of Russia // in History of Organic Chemistry at Russian Universities. Eds. E. Beloglazkina, I. Beletskaya, D. Lewis, V. Nenaydenko. М.: NGB Publishing House. 2022, Р. 272-299.

-
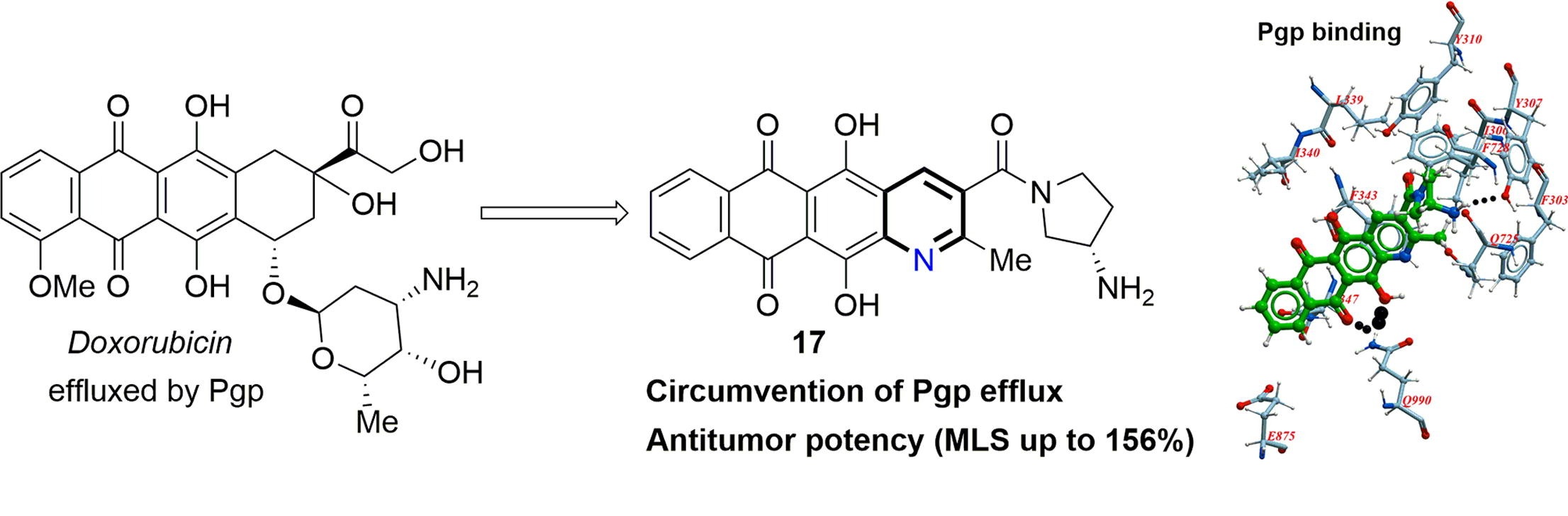
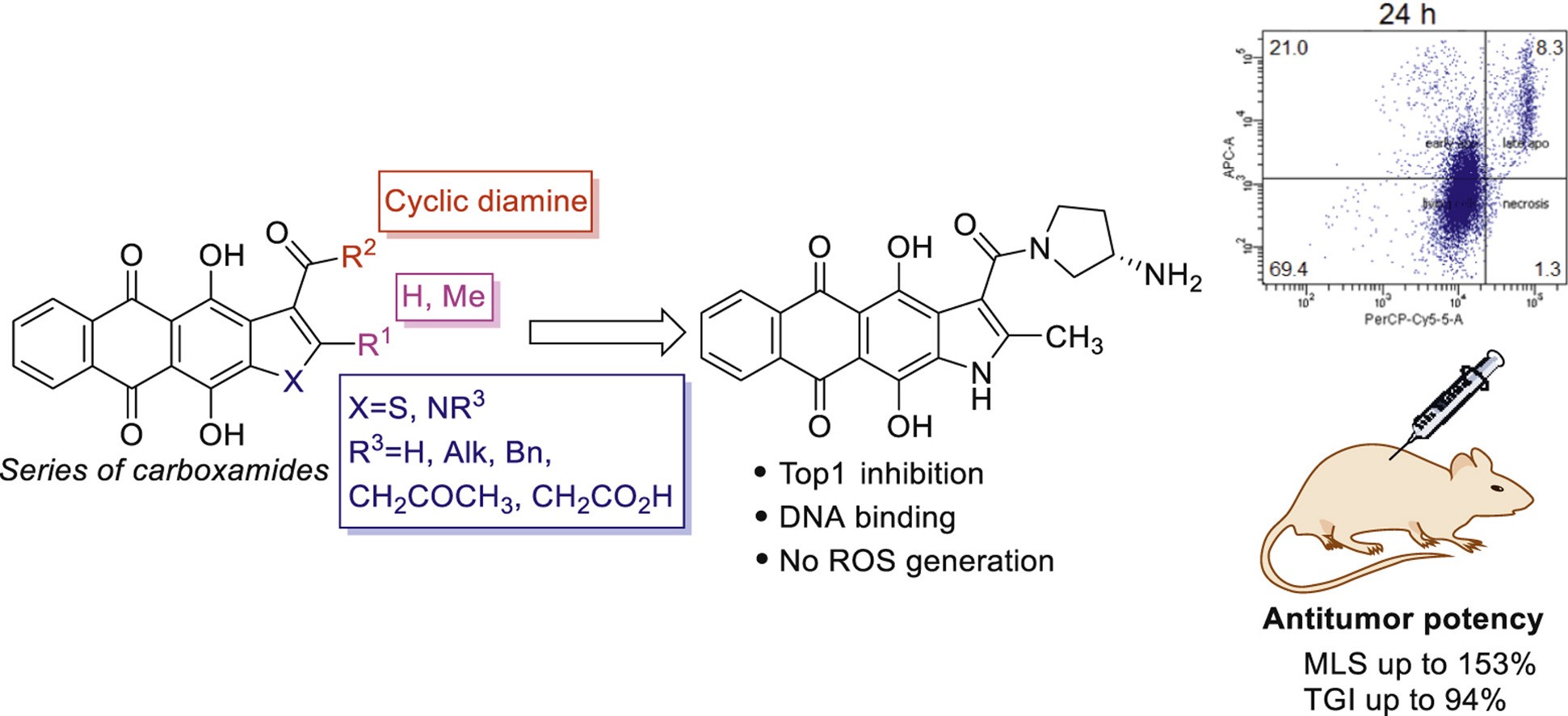
A.S. Tikhomirov, V.B. Tsvetkov, Y.L. Volodina, V.A. Litvinova, D.V. Andreeva, L.G. Dezhenkova, D.N. Kaluzhny, I.D. Treshalin, A.A. Shtil, A.E. Shchekotikhin. Heterocyclic ring expansion yields anthraquinone derivatives potent against multidrug resistant tumor cells, Bioorganic Chemistry, 2022, 127, 105925, https://doi.org/10.1016/j.bioorg.2022.105925
-
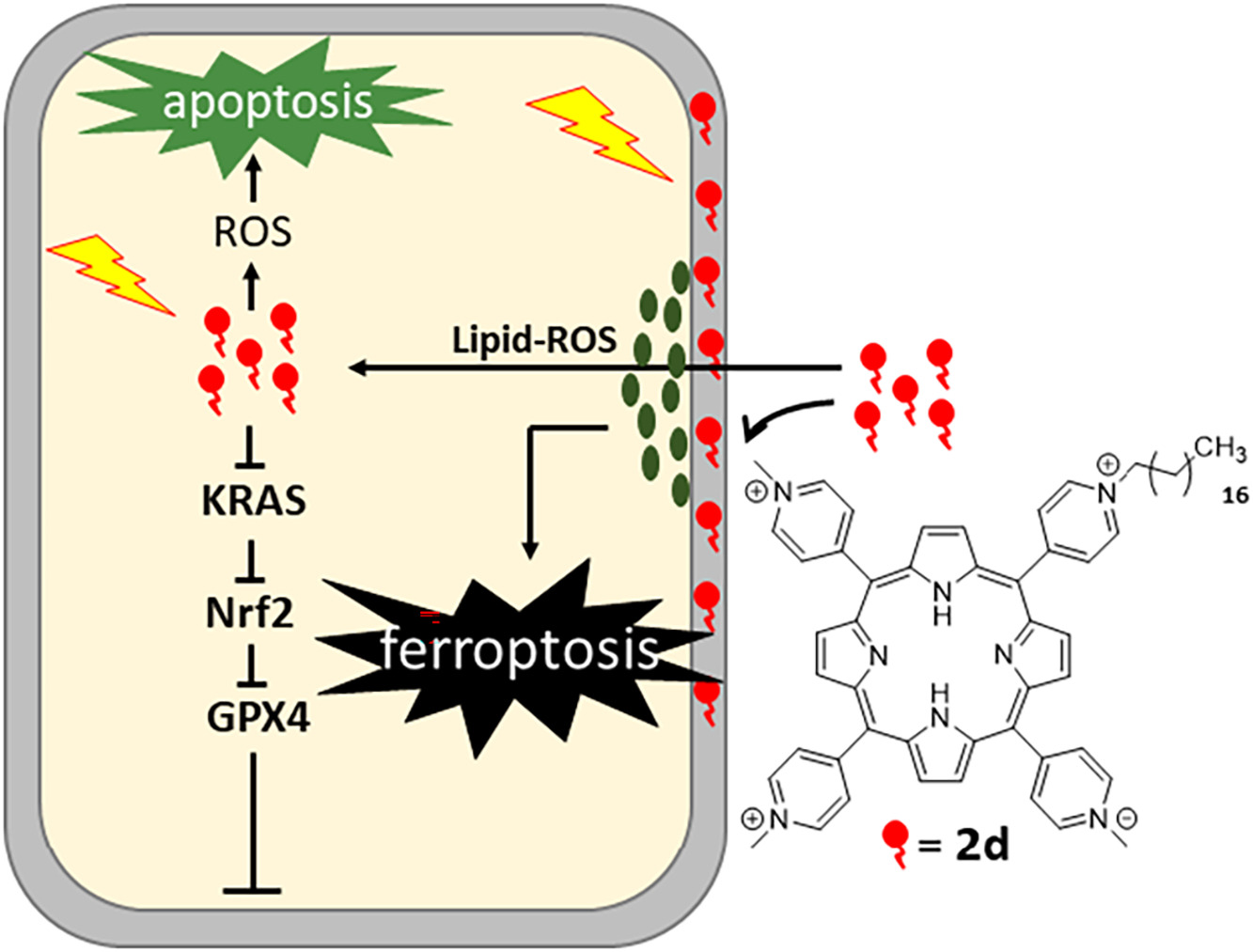
E. Di Giorgio, A. Ferino, H. Choudhary, P. M.G. Löffler, F. D'Este, V. Rapozzi, A. Tikhomirov, A. Shchekotikhin, S. Vogel, L.E. Xodo. Photosensitization of pancreatic cancer cells by cationic alkyl-porphyrins in free form or engrafted into POPC liposomes: The relationship between delivery mode and mechanism of cell death, Journal of Photochemistry and Photobiology B: Biology, 2022, 231, 112449, https://doi.org/10.1016/j.jphotobiol.2022.112449
-
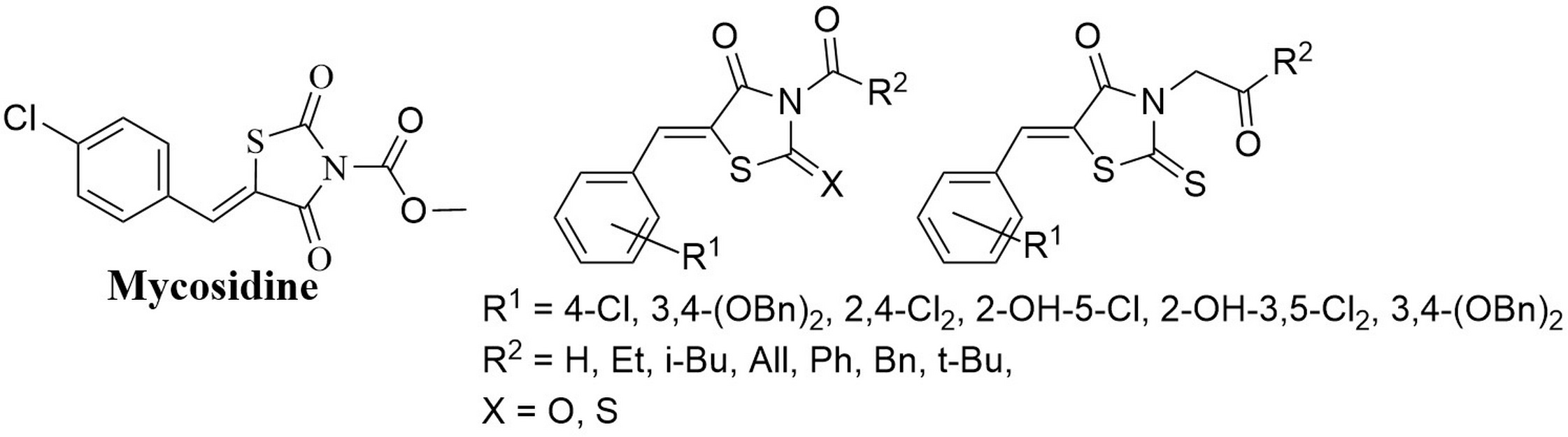
Levshin I.B., Simonov A.Y., Lavrenov S.N., Panov A.A., Grammatikova N.E., Alexandrov A.I., Ghazy E.S.M.O., Savin N.A., Gorelkin P.V., Erofeev A.S., Polshakov V.I. Antifungal Thiazolidines: Synthesis and Biological Evaluation of Mycosidine Congeners. Pharmaceuticals, 2022, 15, 5, 563-586. DOI: 10.3390/ph15050563
-
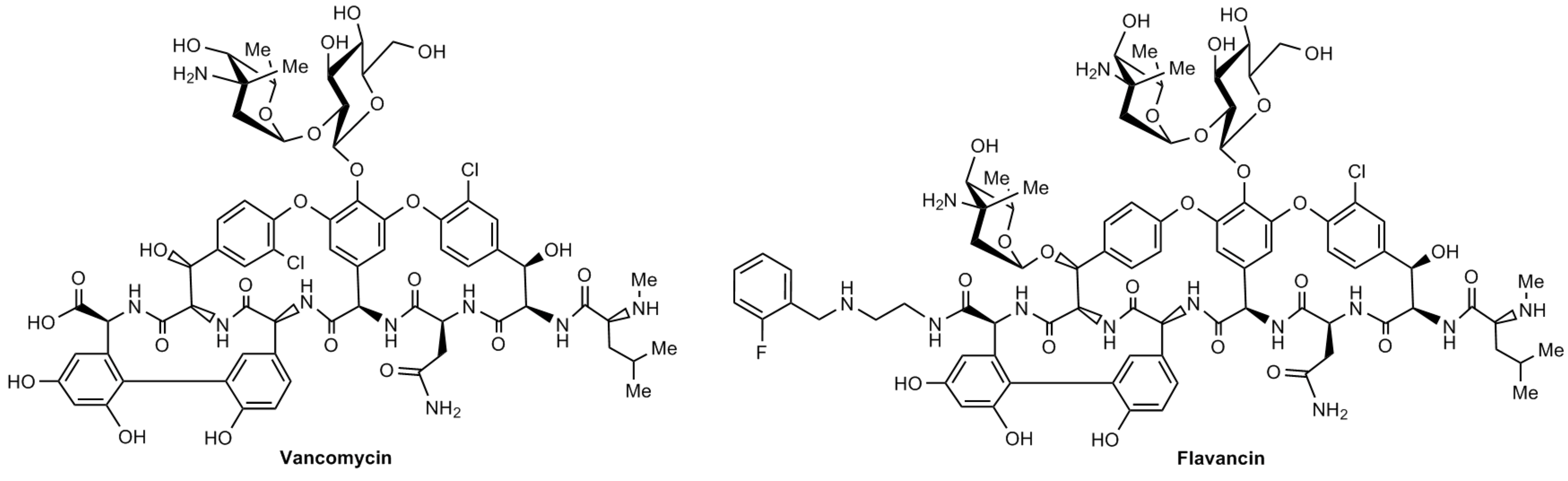
Treshchalin M.I., Polozkova V.A., Moiseenko E.I., Treshalina H.M., Shchekotikhin A.E., Pereverzeva E.R. Evaluation of Toxic Properties of New Glycopeptide Flavancin on Rats. Pharmaceuticals, 2022, 15, 661. DOI: 10.3390/ph15060661
-
Buravchenko G.I. Maslov D.A., Alam M.S., Grammatikova N.E., Frolova S.G., Vatlin A.A., Tia, X., Ivanov I.V., Bekker O.B., Kryakvin M.A., Dontsova O.A., Danilenko V.N., Zhang T., Shchekotikhin A.E. Synthesis and Characterization of Novel 2-Acyl-3-trifluoromethylquinoxaline 1,4-Dioxides as Potential Antimicrobial Agents. Pharmaceuticals 2022, 15(2), 155, https://doi.org/10.3390/ph15020155
-
M.H. Lin, A. Islam, Y.-H. Liu, C.-W. Weng, J.-H. Zhan, R.-H. Liang, A.S. Tikhomirov, A.E. Shchekotikhin, P.J. Chueh, Antibiotic heliomycin and its water-soluble 4-aminomethylated derivative provoke cell death in T24 bladder cancer cells by targeting sirtuin 1 (SIRT1). American Journal of Cancer Research 2022, 12(3), 1042-1055, www.ajcr.us /ISSN:2156-6976/ajcr0140356
-
Perera W.H., Scherbakov A.M., Buravchenko G.I., Mikhaevich E.I., Guimar S., Cos P., Shchekotikhin A.E., Monzote L., Setzer W.N. In Vitro Pharmacological Screening of Essential Oils from Baccharis parvidentata and Lippia origanoides Growing. Molecules, 2022, 27, 1926, https://doi.org/10.3390/molecules27061926
-
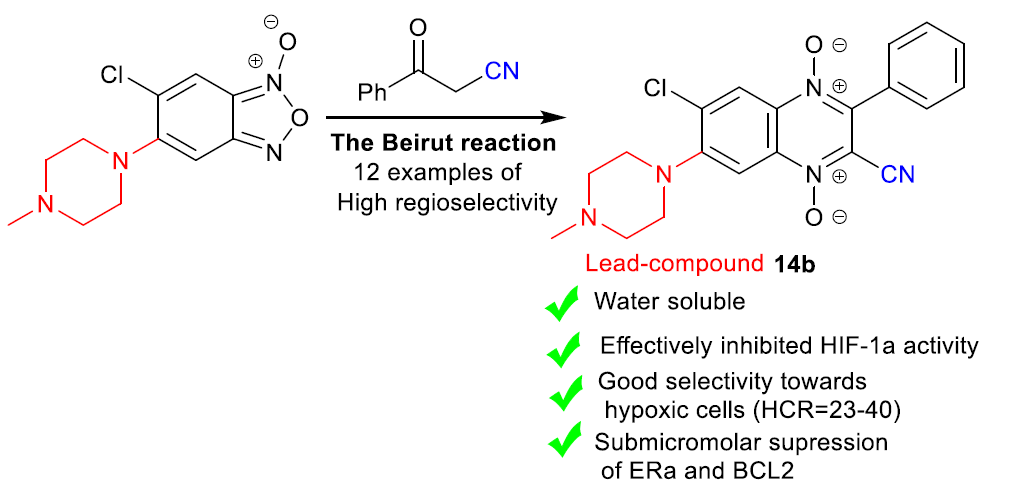
Buravchenko G.I., Scherbakov A.M., Dezhenkova L.G., Fidalgo L., Shchekotikhin, A.E. Synthesis of 7-Amino-6-Halogeno-3-Phenylquinoxaline-2-Carbonitrile 1,4-Dioxides: A Way Forward for Targeting Hypoxia and Drug Resistance of Cancer Cells, RSC Advances, 2021, 11, 38782, doi:10.1039/d1ra07978f.
-
Krymov S.K., Scherbakov A.M., Salnikova D.I., Sorokin D.V., Dezhenkova L.G., Ivanov I.V., Vullo D., De Luca V., Capasso C., Supuran C.T., Shchekotikhin A.E., Synthesis, biological evaluation, and in silico studies of potential activators of apoptosis and carbonic anhydrase inhibitors on isatin-5-sulfonamide scaffold. European Journal of Medicinal Chemistry, 2021, 228, 113997, https://doi.org/10.1016/j.ejmech.2021.113997
-
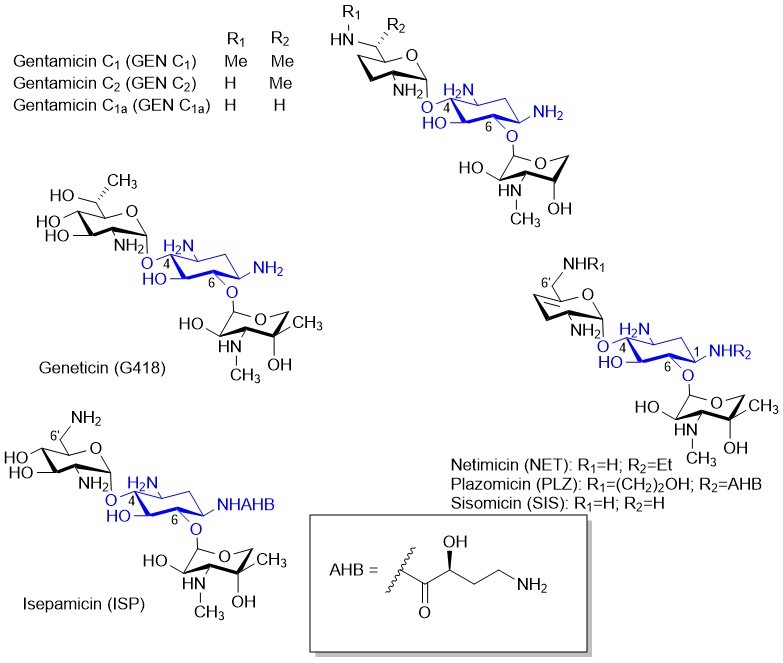
Тевяшова А.Н., Шаповалова К.С. Потенциал разработки аминогликозидных антибиотиков нового поколения. Химико-фармацевтический журнал 2021, 55, 7–23 https://doi.org/10.30906/0023-1134-2021-55-9-7-23
-
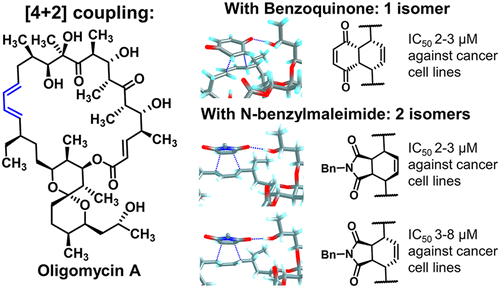
Omelchuk O.A., Malyshev V.I., Medvedev M.G., Lysenkova L.N., Belov N.M., Dezhenkova L.G., Grammatikova N.E., Scherbakov A.M., Shchekotikhin A.E. Stereochemistries and Biological Properties of Oligomycin A Diels–Alder Adducts. Journal of Organic Chemistry 2021, 86, 12, 7975–7986. DOI: 10.1021/acs.joc.1c00296.
-
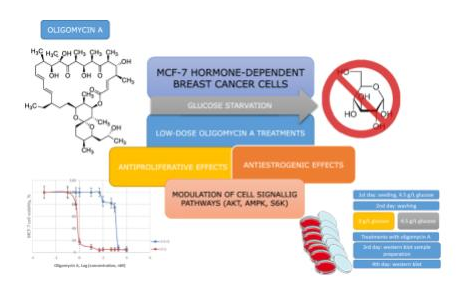
Scherbakov A.M., Sorokin D.V., Omelchuk O.A., Shchekotikhin A.E., Krasil'nikov M.A. Glucose starvation greatly enhances antiproliferative and antiestrogenic potency of oligomycin A in MCF-7 breast cancer cells. Biochimie 2021, 186, 51–58. DOI: 10.1016/j.biochi.2021.04.003.
-
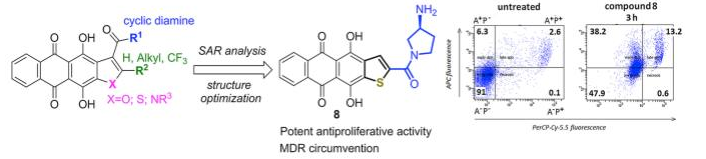
Volodina Y.L., Tikhomirov A.S., Dezhenkova L.G., Ramonova A.A., Kononova A. V., Andreeva D. V., Kaluzhny D.N., Schols D., Moisenovich M.M., Shchekotikhin A.E., Shtil A.A. Thiophene-2-carboxamide derivatives of anthraquinone: A new potent antitumor chemotype. European Journal of Medicinal Chemistry 2021, № 221, 113521. DOI: 10.1016/j.ejmech.2021.113521.
-
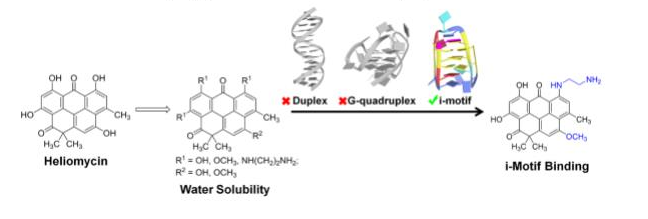
Tikhomirov A.S., Abdelhamid M.A.S., Nadysev G.Y., Zatonsky G.V., Bykov E.E., Chueh P.J., Waller Z.A.E., Shchekotikhin A.E. Water-Soluble Heliomycin Derivatives to Target i-Motif DNA. Journal of Natural Products 2021, 84(5), 1617-1625. doi:10.1021/acs.jnatprod.1c00162.
-
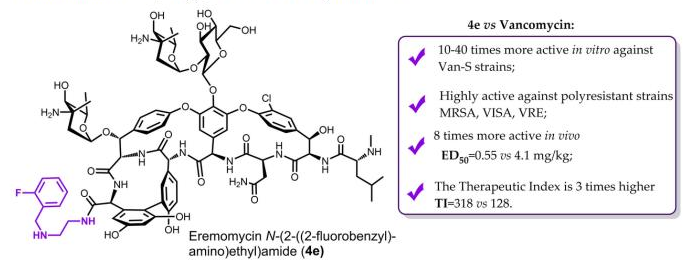
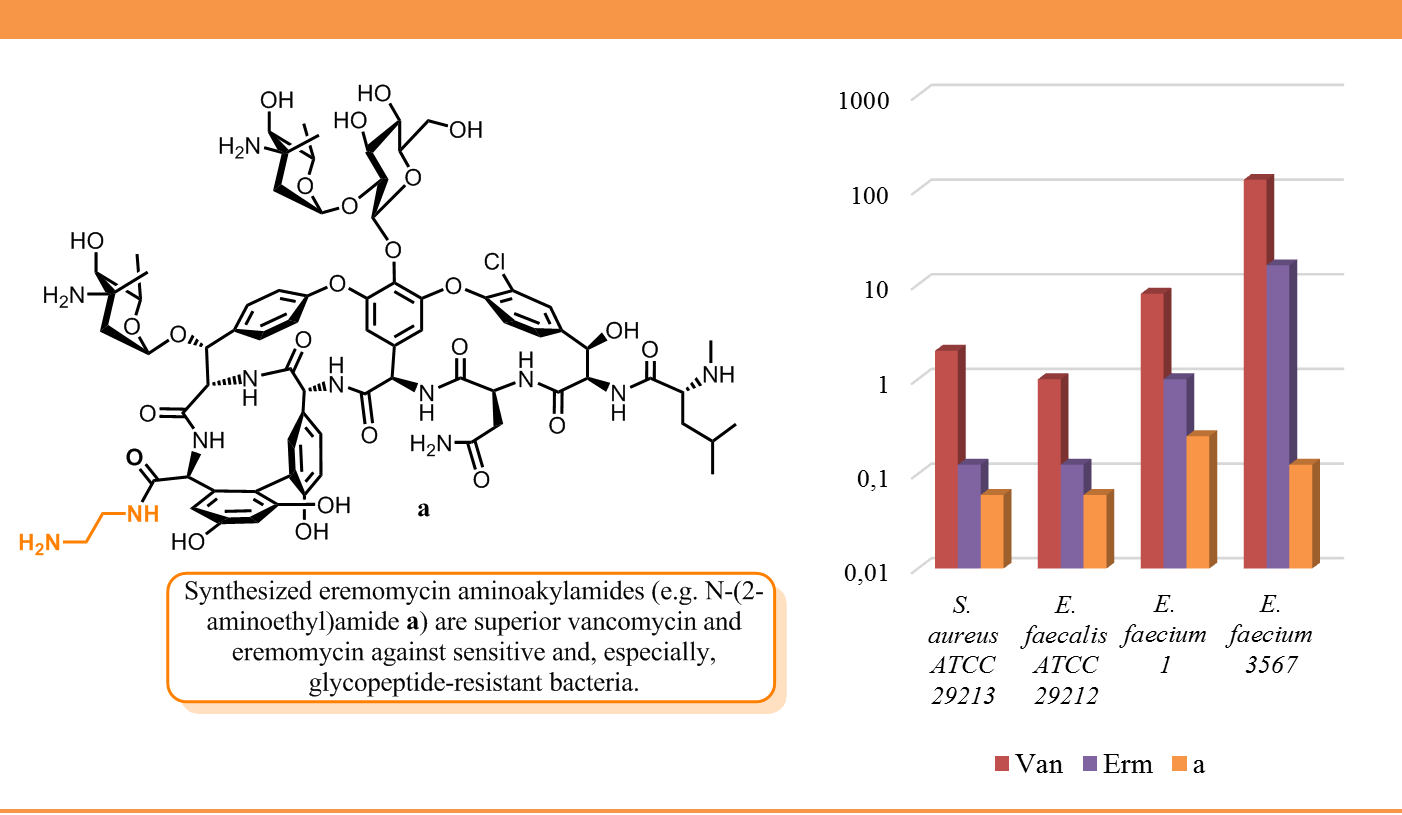
Moiseenko E.I., Erdei R., Grammatikova N.E., Mirchink E.P., Isakova E.B., Pereverzeva E.R., Batta G., Shchekotikhin A.E. Aminoalkylamides of Eremomycin Exhibit an Improved Antibacterial Activity. Pharmaceuticals 2021, 14(4), 379. doi:10.3390/ph14040379.
-
Blokhina S.V., Ol'khovich M. V., Sharapova A. V., Levshin I. B., Perlovich G. L. Study of dissolution and transfer processes of new bioactive thiazolo[4,5-d]pyrimidine derivatives in modeling biological systems. Journal of Molecular Liquids 2021, 337, 116395. doi:10.1016/j.molliq.2021.116395
-
Volkova T. V., Simonova O. R., Levshin I. B., Perlovich G.L. Physicochemical profile of new antifungal compound: pH-dependent solubility, distribution, permeability and ionization assay. Journal of Molecular Liquids 2021, 116535 doi.org/10.1016/j.molliq.2021.116535.
-
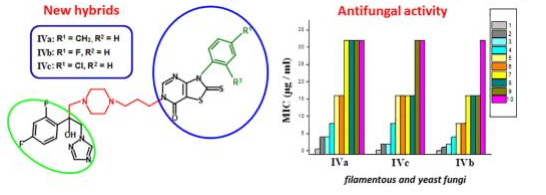
Blokhina S. V., Sharapova A. V., Ol'khovich M. V., Doroshenko I. A., Levshin I. B., Perlovich G. L. (). Synthesis and antifungal activity of new hybrids thiazolo[4,5-d]pyrimidines with (1H-1,2,4)triazole. Bioorganic and Medicinal Chemistry Letters 2021, 40, 127944. doi.org/10.1016/j.bmcl.2021.127944
-
Izsépi L., Erdei R., Tevyashova A.N., Grammatikova N.E., Shchekotikhin A.E., Herczegh P., Batta G. Bacterial cell wall analogue peptides control the oligomeric states and activity of the glycopeptide antibiotic Eremomycin: solution NMR and antimicrobial studies. Pharmaceuticals 2021, 14(2), 83; https://doi.org/10.3390/ph14020083
-
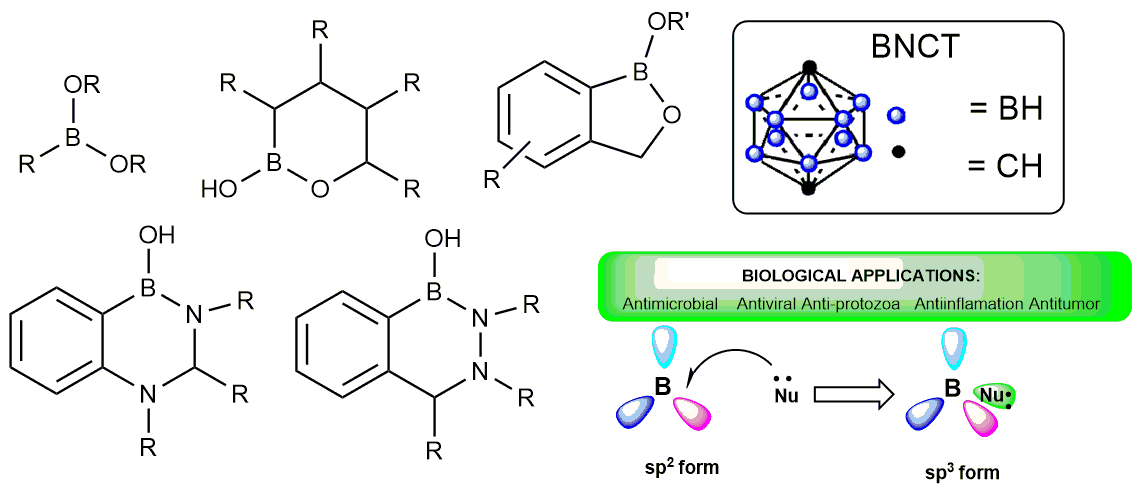
Тевяшова А.Н., Чудинов М.В. Прогресс в медицинской химии органических соединений бора. Успехи химии 2021, 90(4), 451–487; https://doi.org/10.1070/RCR4977
-
Tevyashova A.N. Recent trends in synthesis of chloramphenicol new derivatives. Antibiotics 2021, 10, 370 https://doi.org/10.3390/antibiotics10040370
-
Litvinova V.А., Tikhomirov A.S. New methods for synthesis of 1-benzothiophene-3-carboxylic acid derivatives (microreview). Chemistry of Heterocycic Compounds 2021, 57(2), 131–133. DOI: https://doi.org/10.1007/s10593-021-02882-x.
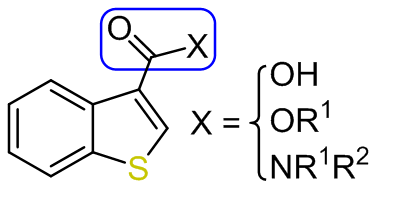
-
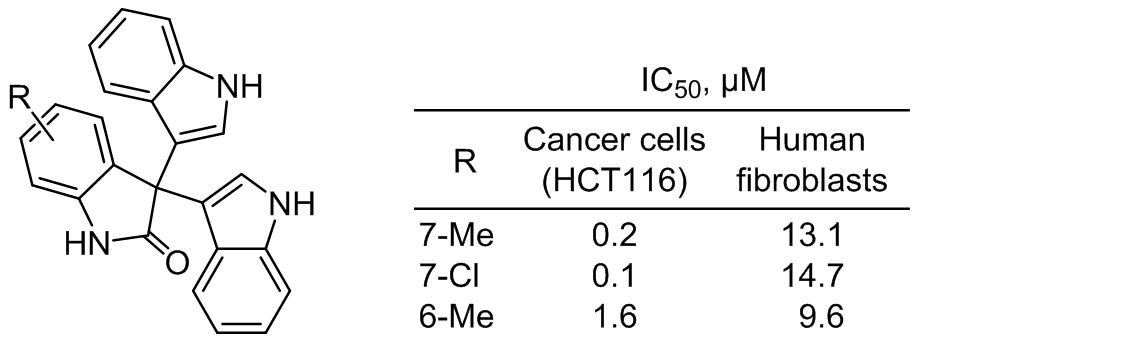
Singh M., Malhotra L., Haque M.A., Kumar M., Tikhomirov A.S, Litvinova V. A, Korolev A.M., Ethayathulla A.S., Das U., Shchekotikhin A.E., Kaur P. Heteroarene-fused anthraquinone derivatives as potential modulators for human aurora kinase B. Biochimie 2021, 182, 152-165. DOI: https://doi.org/10.1016/j.biochi.2020.12.024
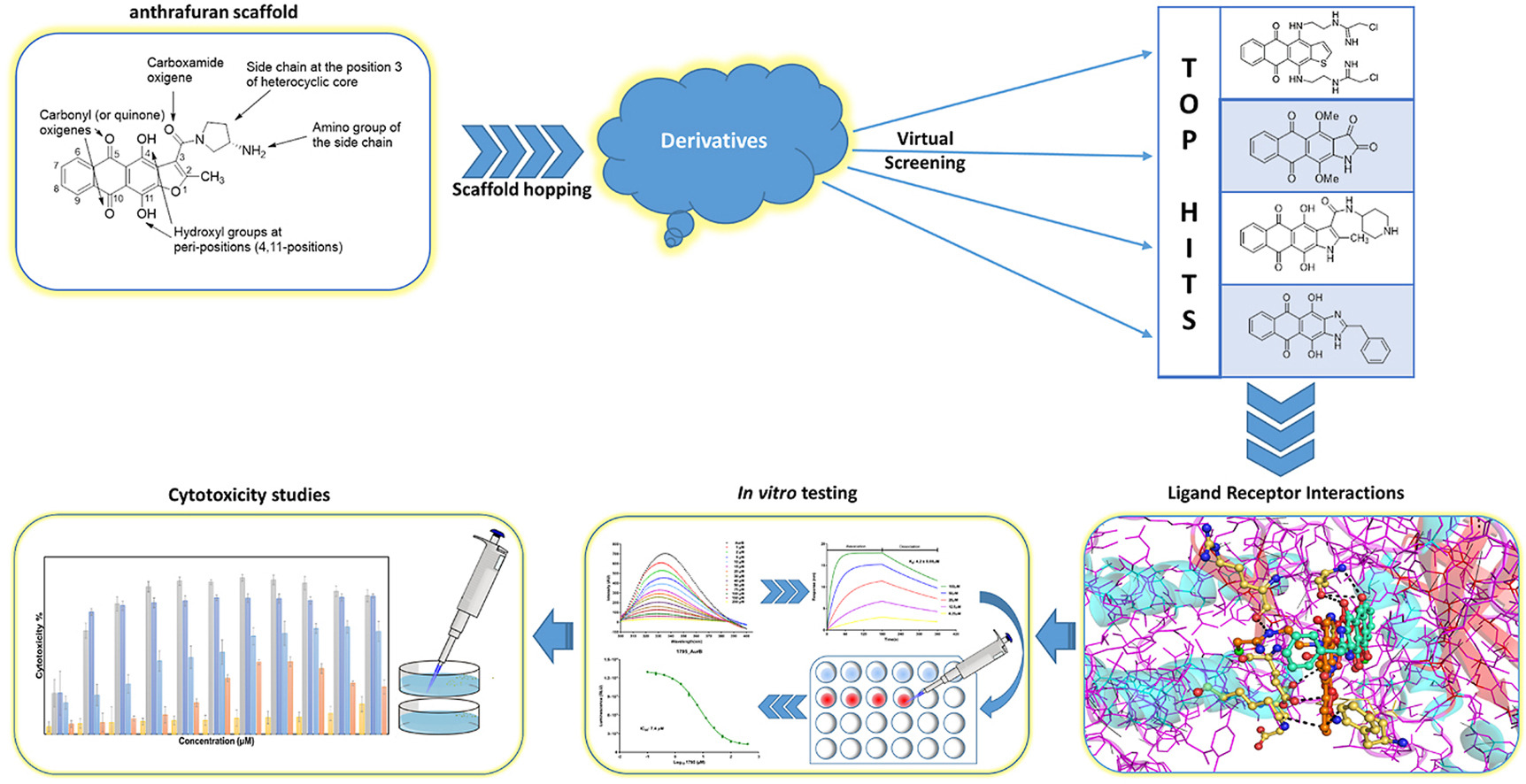
- Buravchenko G.I., Scherbakov A.M., Dezhenkova L.G., Bykov E.E., Solovieva S.E., Korlukov A.A., Sorokin D.V., Monzote Fidalgo L, Shchekotikhin A.E. Discovery of derivatives of 6(7)-amino-3-phenylquinoxaline-2-carbonitrile 1,4-dioxides: novel, hypoxia-selective HIF-1α inhibitors with strong antiestrogenic potency. Bioorganic Chemistry 2020, 104, 104324, doi: 10.1016/j.bioorg.2020.104324.
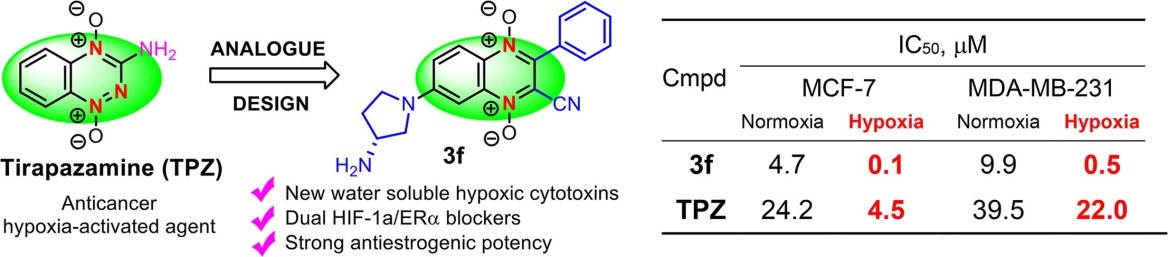
- Monzote L., Scherbakov A.M., Scull R., Satyal P., Cos P., Shchekotikhin A.E., Gille L., Setzer W.N. Essential Oil from Melaleuca leucadendra: Antimicrobial, Antikinetoplastid, Antiproliferative and Cytotoxic Assessment. Molecules 2020, 25, 5514, doi: 10.3390/molecules25235514.
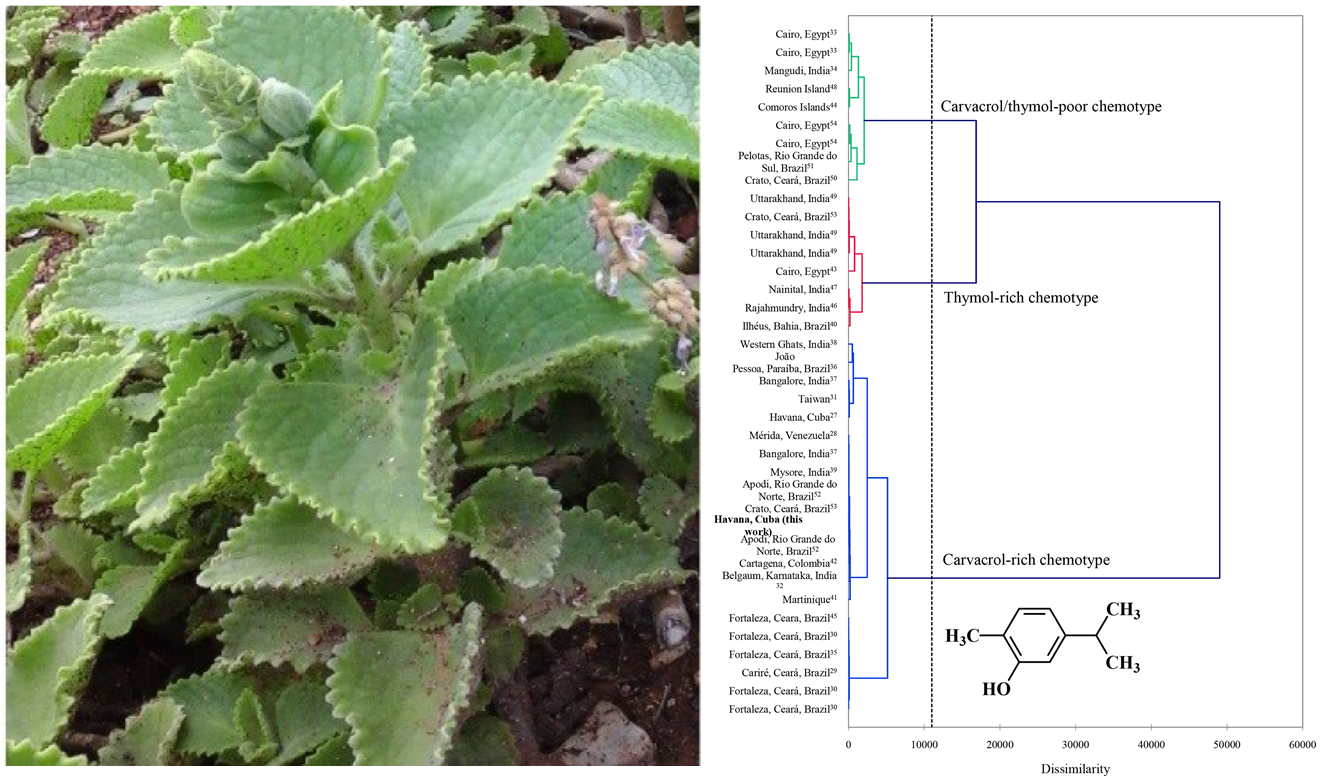
- Monzote L., Scherbakov A. M., Scull R., Gutierrez Y. I., Satyal P., Cos P., Shchekotikhin A. E., Gille L., Setzer W. N. Pharmacological Assessment of the Carvacrol Chemotype Essential Oil From Plectranthus amboinicus Growing in Cuba. Natural Product Communications 2020, 15, № 10, 1–12, DOI: 10. 1177/ 1934 578X 20962233.
- Моисеенко Е. И., Грамматикова Н. Э., Щекотихин А. Е. Синтез и антибактериальная активность аминоалкиламидов эремомицина. Макрогетероциклы 2020, Т. 13, № 3, 298-304, DOI: 10.6060/mhc200812s
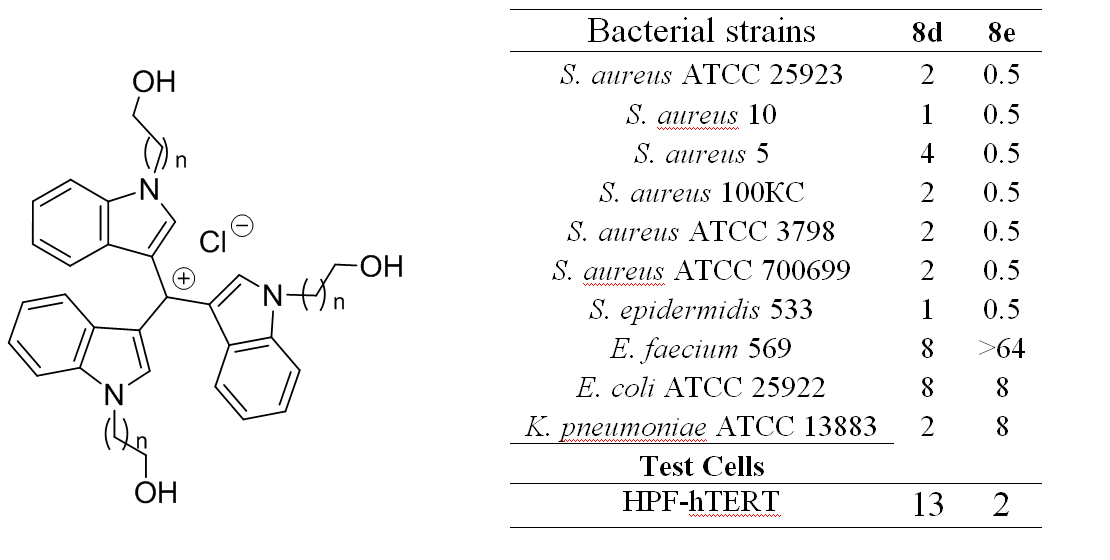
- Lavrenov S.N., Isakova E.B., Panov A.A., Simonov A.Y., Tatarsky V.V., Trenin A.S. N-(Hydroxyalkyl) Derivatives of tris(1H-indol-3-yl)methylium salts as promising antibacterial agents: Synthesis and biological evaluation. Pharmaceuticals 2020, 13 (12), 469, https://doi.org/10.3390/ph13120469
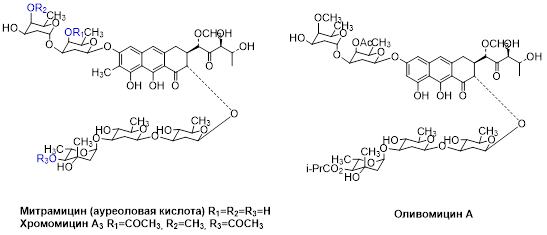
- Bespyatykh J., Bespiatykh D., Malakhova M., Klimina K., Bespyatykh A., Varizhuk A., Tevyashova A., Nikolenko T., Pozmogova G., Ilina E., Shitikov E. Aureolic Acid Group of Agents as Potential Antituberculosis Drugs. Antibiotics 2020, 9(10), 715-730. DOI: 10.3390/antibiotics9100715
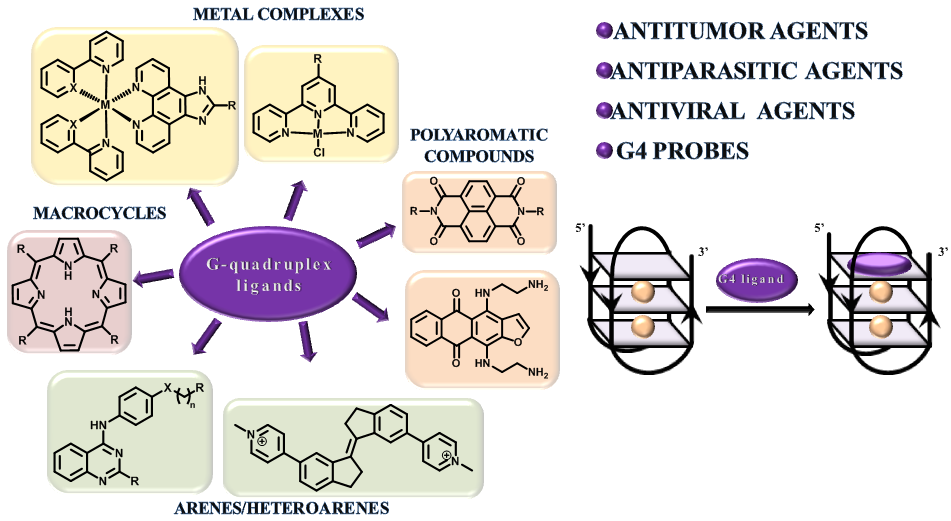
- D.V. Andreeva, A.S. Tikhomirov, A.E. Shchekotikhin. Ligands of G-quadruplex nucleic acids, Russian Chemical Reviews 2021, 90(1), 1-38, DOI: 10.1070/RCR4968
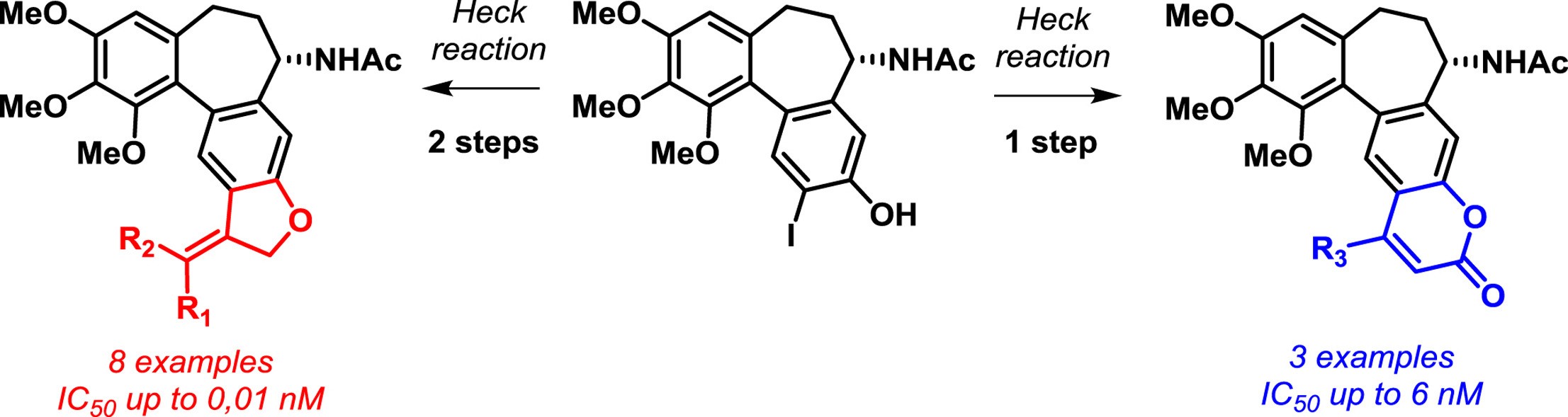
- Shchegravina E.S., Svirshchevskay E.V., Combes S., Allegro D., Barbier P., Gigant B., Varela P.F., Gavryushin A.E., Kobanova D.A., Shchekotikhin A.E., Fedorov A.Yu. Discovery of dihydrofuranoallocolchicinoids - Highly potent antimitotic agents with low acute toxicity. European Journal of Medicinal Chemistry, 2020, 207, 112724. doi: 10.1016/j.ejmech.2020.112724.
- Popov A., Klimovich A., Styshova O., Moskovkina T., Shchekotikhin A., Grammatikova N., Dezhenkova L., Kaluzhny D., Deriabin P., Gerasimenko A., Udovenko A., Stonik V. Design, synthesis and biomedical evaluation of mostotrin, a new water soluble tryptanthrin derivative. International Journal of Molecular Medicine, 2020, 46(4), P. 1335-1346. DOI: 10.3892/ijmm.2020.4693
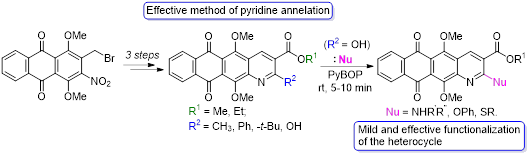
- Litvinova V.A., Tikhomirov A.S., Ivanov I.V., Solovieva S.E., Shchekotikhin A.E. A facile access to 2-substituted naphtho[2,3-g]quinoline-3-carboxylic acid esters via intramolecular cyclization and PyBOP-promoted functionalization. Tetrahedron, 2020, 76(36), 131418. https://doi.org/10.1016/j.tet.2020.131418
-
Andreeva D.V., Tikhomirov A.S., Dezhenkova L.G., Kaluzhny D.N., Mamaeva O.K., Solovyova S.E., Sinkevich Y.B., Shchekotikhin A.E. Heterocyclic analogs of 5,12-naphthacenequinone 16. Synthesis and properties of new DNA ligands based on 4,11-diaminoanthra[2,3-b]thiophene-5,10-dione. Chemistry of Heterocyclic Compounds, 2020, 56(6), 727–733, DOI: 10.1007/s10593-020-02723-3
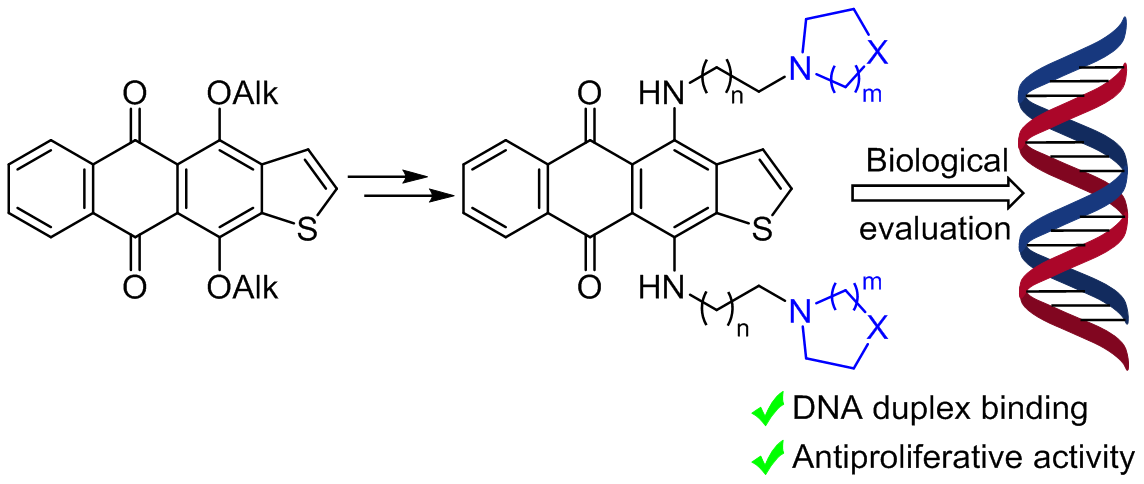
- Vasin A.G., Dezhenkova L.G., Ivanov I.V., Scherbakov A.M., Shchekotikhin A.E. Synthesis and antiproliferative activity of salicylidenehydrazones based on indole-2(3)-carboxylic acids. Chemistry of Heterocyclic Compounds, 2020, 56(6), 734–740, DOI: 10.1007/s10593-020-02724-2
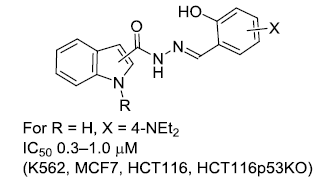
- Lavrenov S.N., Bychkova O.P., Dezhenkova L.G., Mkrtchyan A.S., Tatarskiy V.V., Tsvigun E.A., Trenin A.S. Synthesis and study of cytotoxic activity of novel 3,3-bis(indol-3-yl)-1,3-dihydroindol-2-ones. Chemistry of Heterocyclic Compounds, 2020, 56(6), Р.741–746, DOI: 10.1007/s10593-020-02725-1
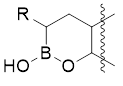
- Tevyashova A.N. Oxaborine derivatives: synthesis and therapeutic potential (minireview). Chemistry of Heterocyclic Compounds, 2020, 56 (6), 715–718. https://doi.org/10.1007/s10593-020-02720-6
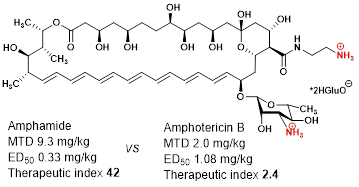
- Tevyashova A.N., Bychkova E.N., Solovieva S.E., Zatonsky G.V., Grammatikova N.E., Isakova E.B., Mirchink E.P., Treshchalin I.D., Pereverzeva E.R., Bykov E.E., Efimova S.S., Ostroumova O.S., Shchekotikhin A.E. Discovery of Amphamide, a Drug Candidate for the Second Generation of Polyene Antibiotics. ACS Infectious Diseases, 2020, 6, 2029−2044 https://dx.doi.org/10.1021/acsinfecdis.0c00068
- Beniaminov A.D., Chashchina G.V., Livshits M.A., Kechko O.I., Mitkevich V.A., Mamaeva O.K., Tevyashova A.N., Shtil A.A., Shchyolkina A.K., Kaluzhny D.N. Discrimination between G/C Binding Sites by Olivomycin A Is Determined by Kinetics of the Drug-DNA Interaction. International Journal of Molecular Sciences, 2020, 21(15), 5299 https://doi.org/10.3390/ijms21155299
- Kuliabina E.V., Tevyashova A.N., Solov’eva S.E., Melkova O.N., Guskova E.A. Reference Materials of Composition of Biologically Active Substances. Measurement Techniques, 2020, 63(4), 325-331 https://doi.org/10.1007/s11018-020-01790-4.
- Tikhomirov A.S., Litvinova V.A., Andreeva D.V., Tsvetkov V.B., Dezhenkova L.G., Volodina Y.L., Kaluzhny D.N.,Treshalin I.D., Schols D. Ramonova A.A., Moisenovich M.M., Shtil A.A., Shchekotikhin A.E. Amides of pyrrole- and thiophene-fused anthraquinone derivatives: A role of the heterocyclic core in antitumor properties. European Journal of Medicinal Chemistry, 2020, 199, 112294. https://doi.org/10.1016/j.ejmech.2020.112294.
- Sagnou M., Novikov F.N., Ivanova E.S., Alexiou P., Stroylov V.S., Titov I.Y., Tatarskiy V.V., Vagida M. S., Pelecanou M., Shtil A.A., Chilov G.G. Novel curcumin derivatives as P-glycoprotein inhibitors: Molecular modeling, synthesis and sensitization of multidrug resistant cells to doxorubicin. European Journal of Medicinal Chemistry, 2020, 198, 112331, https://doi.org/10.1016/j.ejmech.2020.112331
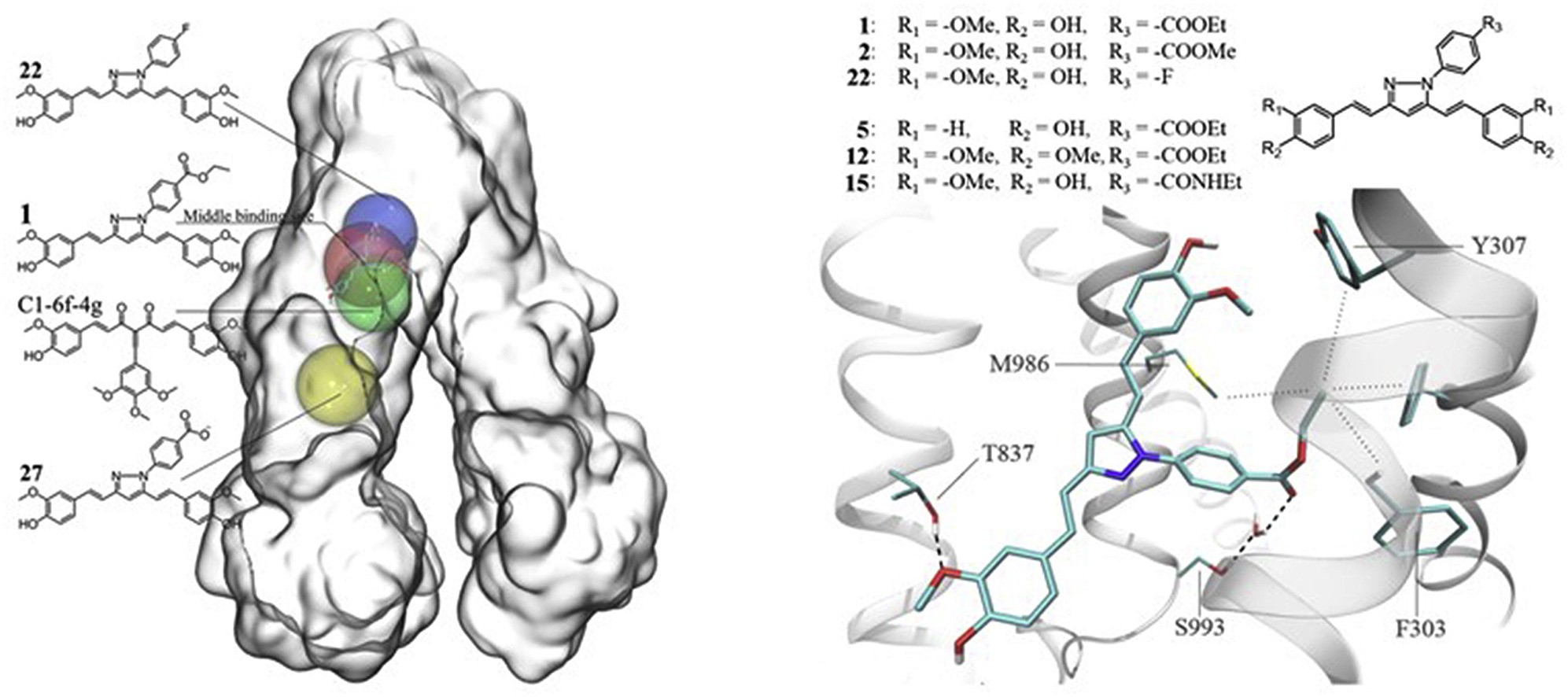
- Shchekotikhin A.E., Treshalina H.M., Treshchalin M.I., Pereverzeva E.R.,Isakova H.B., Tikhomirov A.S. Experimental Evaluation of Anticancer efficiency and acute toxicity of anthrafuran for oral administration, Pharmaceuticals 2020, 13(5), 81, https://doi.org/10.3390/ph13050081
-
Vatlin A.A., Bekker O.B., Lysenkova L.N., Shchekotikhin A.E., Danilenko V.N. Bioinformatics Analysis of Genes of Streptomyces Xinghaiensis (Fradiae) ATCC 19609 With a Focus on Mutations Conferring Resistance to Oligomycin A and Its Derivatives. Journal of Global Antimicrobial Resistance, 2020, 22, 47-53. doi:10.1016/j.jgar.2020.01.026.
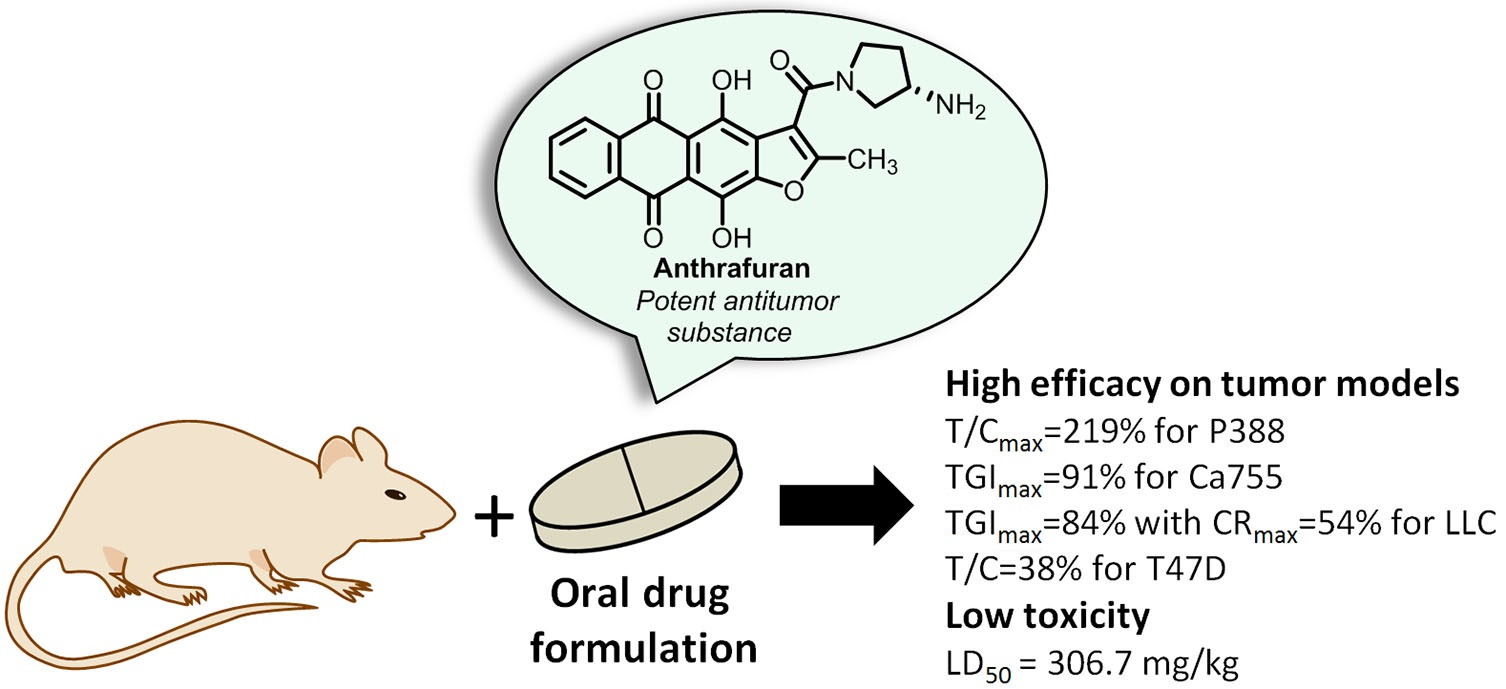
- Buravchenko G.I., Scherbakov А.M., Korlukov A.А., Dorovatovskii P.V., Shchekotikhin A.E. Revision of the regioselectivity of the Beirut reaction of monosubstituted benzofuroxans with benzoylacetonitrile. 6-Substituted quinoxaline-2-carbonitrile 1,4-dioxides: structural characterization and estimation of anticancer activity and hypoxia selectivity. Current Organic Chemistry, 2020, 17, 1-11, DOI: 10.2174/1570179416666191210100754
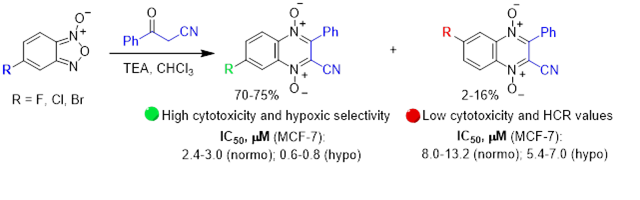
- Ferino A., Nicoletto G., D’Este F. Zorzet S., Lago S., Richter S.N., Tikhomirov A., Shchekotikhin A., Xodo L.E. Photodynamic therapy for ras-driven cancers: targeting g-quadruplex RNA structures with bifunctional alkyl-modified porphyrins. Journal of Medicinal Chemistry, 2020, 63(3), 1245-1260. https://doi.org/10.1021/acs.jmedchem.9b01577
- Olsufyeva E.N., Yankovskaya V.S., Main trends in the design of semi-synthetic antibiotics of a new generation, Russian Chemical Reviews, 2020, 89(3), 339–378. DOI: https://doi.org/10.1070/RCR4892

-
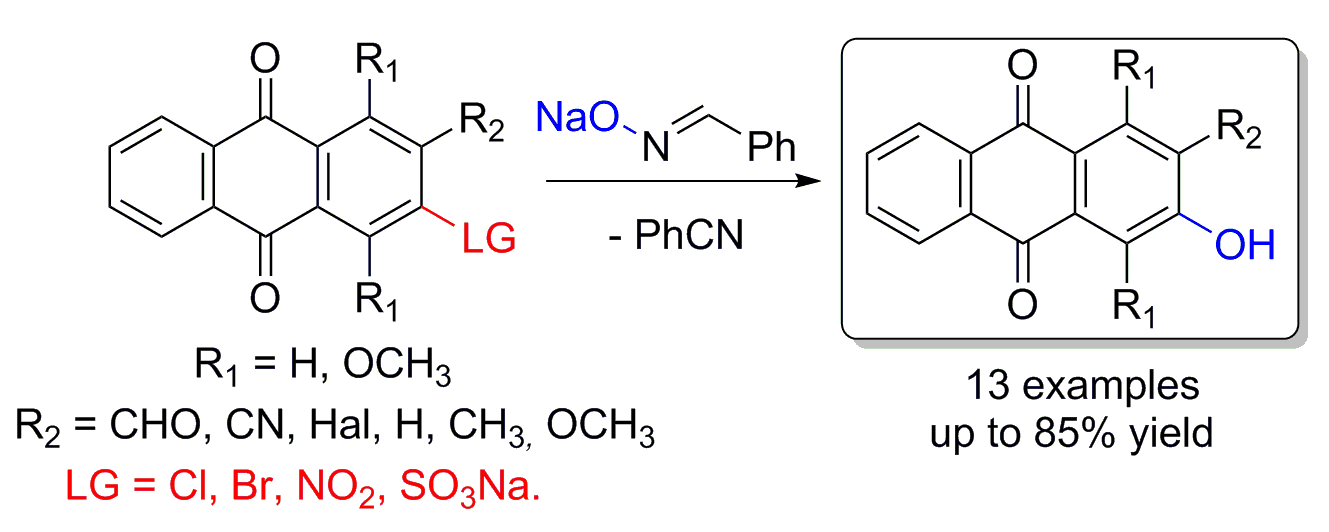
A.S. Tikhomirov, I.V. Ivanov, A.M. Korolev, A.E. Shchekotikhin. β-Hydroxylation of anthraquinone derivatives with benzaldehyde oxime as a source of hydroxyl group. Tetrahedron, 2019, 75, 130623. https://doi.org/10.1016/j.tet.2019.130623
-
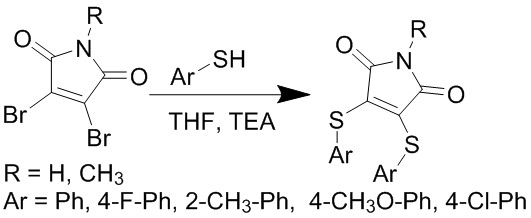
Panov A.A., Lavrenov S.N., Simonov A.Yu., Mirchink E.P., Isakova E.., Trenin A.S. // Synthesis and antimicrobial activity of 3,4-bis(arylthio)maleimides. /J. Antibiot (Tokyo). 2019. V. 71. № 2. P. 122-124. DOI : 10.1038/s41429-018-0122-3.
-
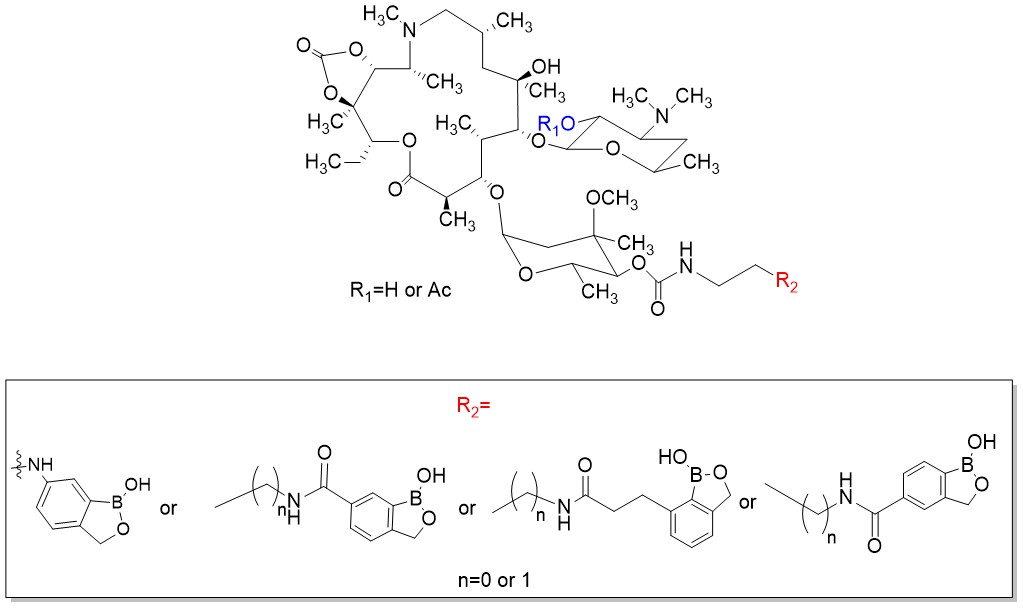
Tevyashova A.N., Korolev A.M., Mirchink E.P., Isakova E.B., Osterman I.A. //Synthesis and evaluation of biological activity of benzoxaborole derivatives of azithromycin. / J. Antibiot (Tokyo). 2019. V. 72. № 1. Р.22-33. DOI: 10.1038/s41429-018-0107-2
-
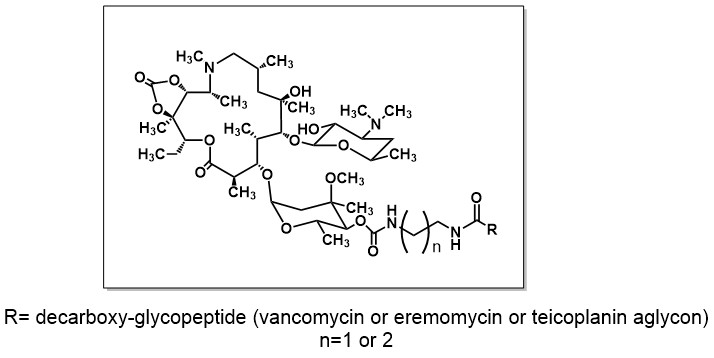
Tevyashova A.N., Bychkova E.N., Korolev A.M., Isakova E.B., Mirchink E.P., Osterman I.A., Erdei R., Szucs. Z., Bata G. // Synthesis and evaluation of biological activity for dual-acting antibiotics on the basis of azithromycin and glycopeptides./ Bioorganic&MedicinalChemistry Letters 2019. V. 29. № 2. P. 276-280. DOI: 10.1016/j.bmcl.2018.11.038 WOS Q2
-
Sergeev A.V., Tevyashova A.N., Vorobyov A.P., Gromova, E.S. // The Effect of Antitumor Antibiotic Olivomycin A and Its New Semi-synthetic Derivative Olivamide on the Activity of Murine DNA Methyltransferase Dnmt3a./ Biochemistry (Moscow). 2019. V. 84. № 1. P. 62-70. DOI: 10.1134/S0320972519020064
-
Bekker O.B., Vatlin A.A., Zakharevich N.V., Lysenkova L.N., Shchekotikhin A.E., Danilenko V.N.// Draft Genome Sequence of Streptomyces xinghaiensis (fradiae) OlgR, a Strain Resistant to Oligomycin A./ Microbiol Resour Announc. 2019. V. 8. № 2. № статьи: e01531-18. DOI: 10.1128/MRA.01531-18.
-
Моисеенко Е.И., Грамматикова Н.Э., Щекотихин А. Е.// Пиколиламиды эремомицина и катионные липогликопептиды на их основе: cинтез и оценка антимикробных свойств./ Макрогетероциклы, 2019. Т. 12. №1. С. 98-106. DOI: 10.6060/mhc181216s
-
Ефимова C.C., Тертычная Т.Е., Лавренов С.Н., Остроумова О.С. // Механизмы действия производных трииндолилметана на липидные мембраны. /Acta Naturae. 2019. Т. 11. № 3 (42). С.38-45. DOI: 10.32607/20758251-2019-11-3-38-45
-
Панов А. А., Лакатош С. A., Куббутат М. Х. Г., Деженкова Л. Г., Тотцке Ф., Шехтеле К. // Новые 3,4-бис(индол-1-ил)малеимиды как ингибиторы протеинкиназ. / Химия гетероциклических соединений. 2019. Т. 55. № 11. Р. 1050–1059.
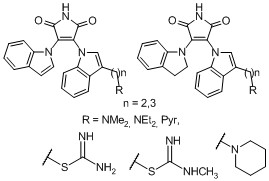
-
Тевяшова А.Н., Бычкова Е.Н., Соловьева С.Е., Грамматикова Н.Э., Щекотихин А.Е.// Разработка парентеральной лекарственной формы нового противогрибкового полусинтетического полиенового антибиотика Амфамида./ Хим.-фарм. журнал. 2019. Т. 53.№10. С. 50-54.
-
Панов А.А., Симонов А.Ю., Королёв А.М. // Синтез новых производных 3-(арилтио)малеимида./ Журнал Органической химии. 2019. Т. 55. № 12. С. 1850–1856. DOI: 10.1134/S0514749219120061
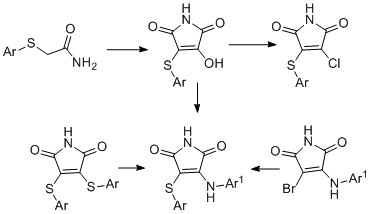
-
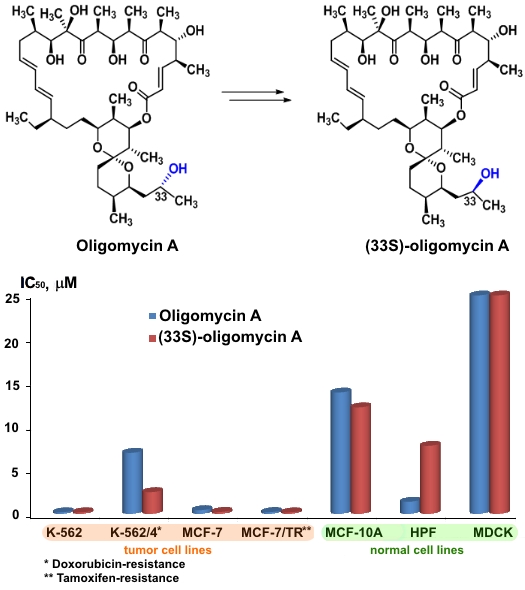
Lysenkova L.N., Saveljev O.Y., Omelchuk O.A., Zatonsky G.V., Korolev A.M., Grammatikova N.E., Bekker O.B., Danilenko V.N., Dezhenkova L.G., Mavletova D.A., Scherbakov A.M., Shchekotikhin A.E. Synthesis, antimicrobial and antiproliferative properties of epi-oligomycin A, the (33S)-diastereomer of oligomycin A // Natural Product Research, DOI: 10.1080/14786419.2019.1608540.
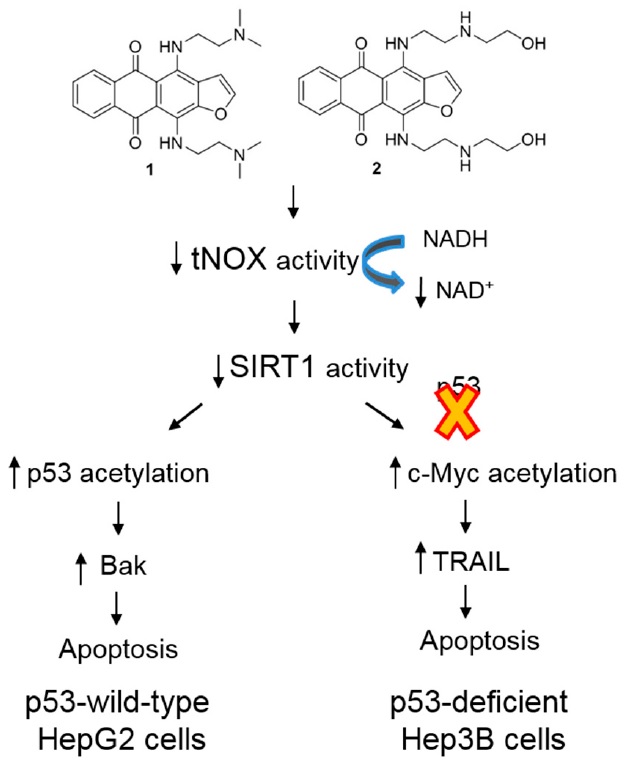
- C.-Y. Lin, A. Islam, C.J. Su, A.S. Tikhomirov, A.E. Shchekotikhin, S.-M. Chuang, P.J. Chueh, Y.L. Chen. Engagement with tNOX (ENOX2) to Inhibit SIRT1 and Activate p53-Dependent and -Independent Apoptotic Pathways by Novel 4,11-Diaminoanthra[2,3-b]furan-5,10-diones in Hepatocellular Carcinoma Cells. Cancers 2019, 11(3), 420. doi: 10.3390/cancers11030420
- Y.L. Volodina, L.G. Dezhenkova, A.S. Tikhomirov, V.V. Tatarskiy, D.N. Kaluzhny, A.M. Moisenovich, M.M. Moisenovich, A.K. Isagulieva, A.A. Shtil, V.B. Tsvetkov, A.E. Shchekotikhin. New anthra[2,3-b]furancarboxamides: A role of positioning of the carboxamide moiety in antitumor properties. European Journal of Medicinal Chemistry 2019, 165, 31-45. https://doi.org/10.1016/j.ejmech.2018.12.068
- Evgenia N. Olsufyeva, Andrey E. Shchekotikhin, Elena N. Bychkova, Eleonora R. Pereverzeva, Ivan D. Treshalin, Elena P. Mirchink1, Elena B. Isakova, Mikhail G. Chernobrovkin, Roman S. Kozlov, Andrey V. Dekhnich, Maria N. Preobrazhenskaya Eremomycin pyrrolidide: a novel semisynthetic glycopeptide with improved chemotherapeutic properties Drug Design, Development and Therapy 2018:12 1–11
- G. Cozza, M. Fortuna, F. Meggio, S. Sarno, M. H. G. Kubbutat, F. Totzke,C. Schaechtele, L. A. Pinna, E. N. Olsufyeva, and M. N. Preobrazhenskaya Hydrophobic Derivatives of Glycopeptide Antibiotics as Inhibitors of Protein Kinases Biochemistry (Moscow), 2018, Vol. 83, No. 10, pp. 1222_1230. Pleiades Publishing, Ltd., 2018. Published in Russian in Biokhimiya, 2018, Vol. 83, No. 10, pp. 1523_1533.
- Д. Коцца, М. Фортуна, Ф. Меггио, С. Сарно, М.Х.Д. Куббутат, Ф. Тотцк, С. Шаехтеле, Л.А. Пинна, Е.Н.Олсуфьева, М.Н. Преображенская Гидрофобные производные гликопептидных антибиотиков как новый класс ингибиторов протеинкиназ БИОХИМИЯ, 2018, том 83, вып. 10, с. 1523 – 1533 DOI: 10.1134/S032097251810007Х
- Tikhomirov A.S., Tsvetkov V.B., Kaluzhny D.N., Volodina Y.L., Zatonsky G.V., Schols D., Shchekotikhin A.E. Tri-armed Ligands of G-Quadruplex on Heteroarene-fused Anthraquinone Scaffolds: Design, Synthesis and Рre-screening of Biological Properties. European Journal of Medicinal Chemistry. 2018, 159, 59-73. https://doi.org/10.1016/j.ejmech.2018.09.054.
- O.A. Omelchuk, N.M. Belov, V.B. Tsvetkov, A.M. Korolev, L.G. Dezhenkova, N.E. Grammatikova, L.N. Lysenkova, O.B. Bekker, V.N. Danilenko, A.E. Shchekotikhin. Synthesis and biological activity of 16,33-O,O-diformyl-16,17-dihydro-16(S),17(R)-dihydroxyoligomycin A and 33-O-formyloligomycin A. Macroheterocycles. 2018. 11(2), 181–192. [DOI: 10.6060/mhc170834o]
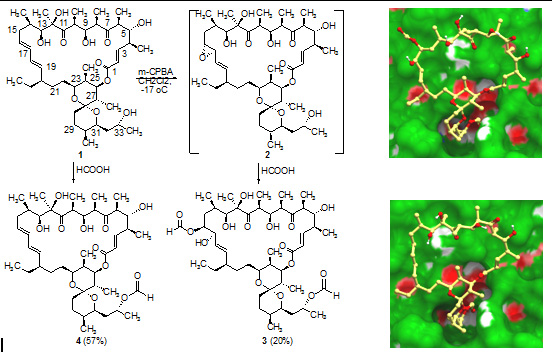
- D.V. Andreeva, Y.B. Sinkevich, A.S. Tikhomirov, Y.N. Luzikov, A.M. Korolev, Y.B. Sinkevich, A.E. Shchekotikhin. Heterocyclic analogs of 5,12-naphtacenequinone 15. Synthesis of new anthra[2,3-b]thiophene-3(2)-carboxylic acids. Chemistry of Heterocyclic Compounds. 2018. № 6, 612-617.
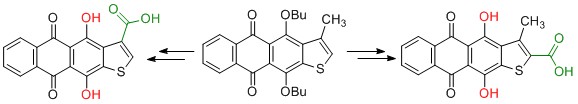
- G.Y. Nadysev, A.S. Tikhomirov, L.G. Dezhenkova, A.E. Shchekotikhin, Semi-Synthetic Derivatives of Heliomycin with an Antiproliferative Potency, Recent Patents on Anti-Cancer Drug Discovery, 2018, 13.DOI:10.2174/1574892813666180723150542.
- A.-C. Schulz-Fincke, A.S. Tikhomirov, A. Braune, T. Girbl, E. Gilberg, J. Bajorath, M. Blaut, S. Nourshargh, M. Gütschow. Design of an Activity-Based Probe for Human Neutrophil Elastase: Implementation of the Lossen Rearrangement To Induce Förster Resonance Energy Transfers. Biochemistry 2018, Vol. 57, 742-752.
- A.S. Tikhomirov, A.A. Shtil, A.E. Shchekotikhin. Advances In the Discovery of Anthraquinone-Based Anticancer Agents. Recent Patents on Anticancer Drug Discovery 2018, 2.DOI:10.2174/1574892813666171206123114 Anticancer Drug Discovery_ 2018
- A.S. Tikhomirov, C.-Y. Lin, Y.L. Volodina, L.G. Dezhenkova, V.V. Tatarskiy, D. A.A. Shtil, P. Kaur, P.J. Chueh, A.E. Shchekotikhin. New Antitumor Anthra[2,3-b]furan-3-carboxamides: Synthesis and Structure-Activity Relationship. European Journal of Medicinal Chemistry European Journal of Medicinal Chemistry_02_18 2018,https://doi.org/10.1016/j.ejmech.2018.02.027
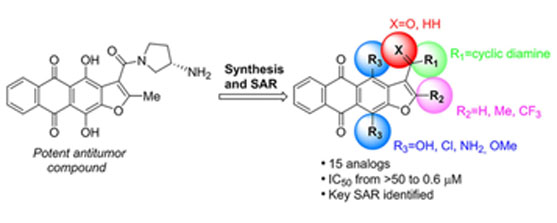
- Svetlana S. Efimova, Anna N. Tevyashova, Evgenia N. Olsufyeva, Evgeny E. Bykov,Olga S. Ostroumova Pore-forming activity of new conjugate antibiotics based on amphotericin B, PLOS ONE | https://doi.org/10.1371/journal.pone.0188573 November 29, 2017, P.1-14
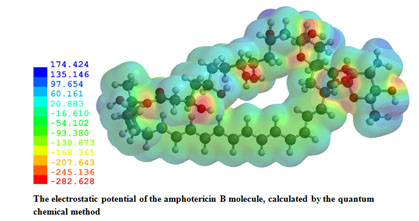
- G.Y. Nadysev, A.S Tikhomirov, M.H. Lin, Y.T. Yang, L.G. Dezhenkova, H.Y. Chen, D.N. Kaluzhny, D. Schols, A.A. Shtil, A.E. Shchekotikhin, P.J. Chueh. Aminomethylation of heliomycin: Preparation and anticancer characterization of the first series of semi-synthetic derivatives. European Journal of Medicinal Chemistry. 2017. https://doi.org/10.1016/j.ejmech.2017.10.055
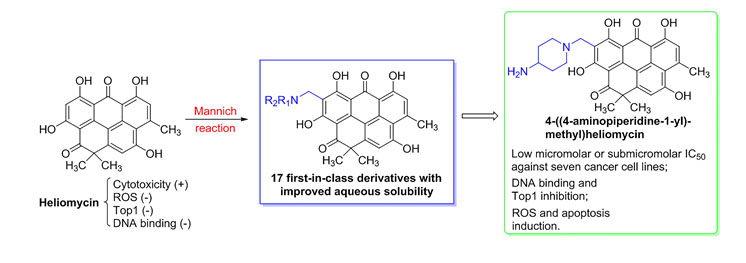
- H.M. Treshalina, V.I. Romanenko, D.N. Kaluzhny, M.I. Treshalin, A.A. Nikitin, A.S. Tikhomirov, A.E. Shchekotikhin. Development and pharmaceutical evaluation of the anticancer Anthrafuran/Cavitron complex, a prototypic parenteral drug formulation. European Journal of Pharmaceutical Sciences. 2017. Vol. 109, 631-637. https://doi.org/10.1016/j.ejps.2017.09.025.
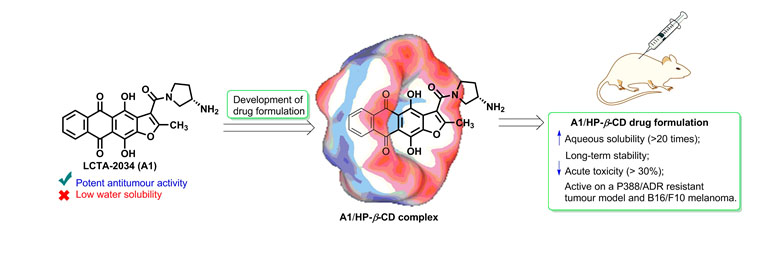
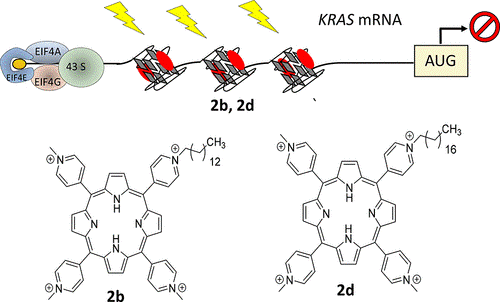
- G. Miglietta, S. Cogoi, J. Marinello, G. Capranico, A.S. Tikhomirov, A. Shchekotikhin, L.E. Xodo. RNA G-Quadruplexes in Kirsten Ras (KRAS) Oncogene as Targets for Small Molecules Inhibiting Translation. Journal of Medicinal Chemistry 2017. DOI: 10.1021/acs.jmedchem.7b00622
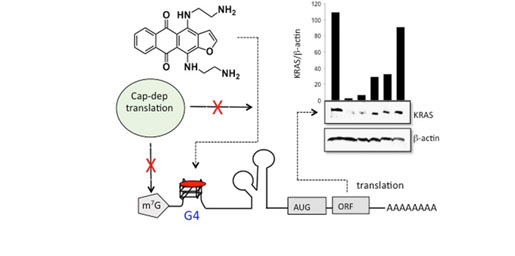
- A.S. Tikhomirov, V.A. Litvinova, Y.N. Luzikov, A.M. Korolev, Y.B. Sinkevich, A.E. Shchekotikhin. Heterocyclic analogs of 5,12-naphtacenequinone 14. Synthesis of naphto[2,3-f]indole-3-carboxylic acid derivatives. Chemistry of Heterocyclic Compounds. 2017. № 10, 1072-1079.
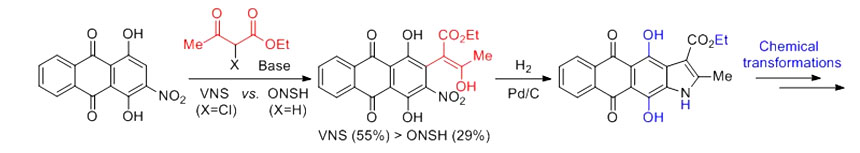
- Быков Е.Е., Мирчинк Е.П., Исакова Е.Б., Бычкова Е.Н., Олсуфьева Е.Н., Тевяшова А.Н.Изучение антибактериальной активности и связывания с пептидным лигандом гибридных антибиотиков ванкомицин-азитромицин и эремомицин-азитромицин "Антибиотики и химиотерапия" 2017, Т.62, №3-4
- Lyudmila N Lysenkova, Oleg Y Saveljev, Natalya E Grammatikova, Vladimir B Tsvetkov, Olga B Bekker, Valery N Danilenko, Lyubov G Dezhenkova, Eugene E Bykov, Olga A Omelchuk, Alexander M Korolev and Andrey E Shchekotikhin Verification of oligomycin A structure: synthesis and biological evaluation of 33-dehydrooligomycin A he Journal of Antibiotics , (19 April 2017) | doi:10.1038/ja.2017.48
- O.A. Omelchuk, N.M. Belov, V.B. Tsvetkov, N.E. Grammatikova, L.N. Lysenkova, A.M. Korolev, O.B. Bekker, V.N. Danilenko, A.E. Shchekotikhin. Synthesis and biological activity of 2,3,16,17,18,19-hexahydrooligomycin A. Macroheterocycles. 2016. 9(4), 453–461. [DOI: 10.6060/mhc160964o]
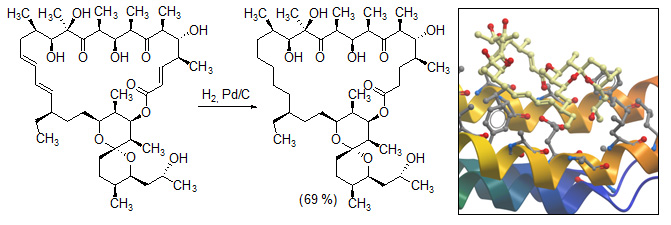
- О.А. Омельчук, А.С.Тихомиров, А.Е. Щекотихин. Методы аннелирования фуранового ядра к аренам. Успехи химии. 2016. 85(8), 817–835. [DOI: 10.1070/RCR4613]
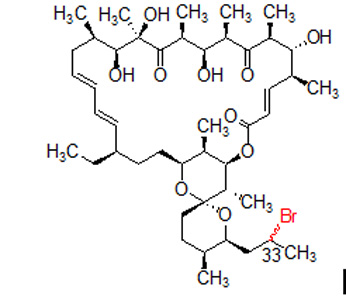
- L.N. Lysenkova, O.Y. Saveljev, A.M. Korolev, V.N. Danilenko, O.B. Bekker, D.A. Mavletova, A.A. Vatlin, O.A. Omelchuk, A.E. Shchekotikhin. Synthesis of 33-(R,S)-Bromo-33-deoxyoligomycin A. Macroheterocycles. 2016. 9(3), 307–313. [DOI: 10.6060/mhc160422s]
- Grinberg V.Y., Tsvetkov V.B., Markova A.A., Dezhenkova L.G., Burova T.V., Grinberg N.V., Dubovik A.S., Plyavnik N.V., Shtil A.A. Interactions of non-phosphorous glycerolipids with DNA: Energetics, Molecular docking and topoisomerase I attenuation. Anti-Cancer Agents in Medicinal Chemistry. 2016. V.16, Р.335-346.
- Chang J., Zhou H., Preobrazhenskaya M., Tao P., Kim S.J. The сarboxyl terminus of eremomycin facilitates binding to the non D-Ala-D-Ala segment of the peptidoglycan pentapeptide stem. Biochemistry. 2016. V.55, №24, Р.3383–3391.
- Принцевская С.С., Королев А.М., Исакова E.Б., Мирчинк E.П., Тевяшова А.Н. Гибридные антибиотики на основе азитромицина и гликопептидов – синтез и антибактериальная активность. Антибиотики и химиотерапия 2016. Т.61, №11-12
- Тевяшова А.Н., Олсуфьева Е.Н., Преображенская М.Н. Создание антибиотиков двойного действия как путь поиска новых перспективных лекарственных препаратов. Успехи химии, 2015, т. 84, стр.61-97.
- M. Singh, S,J. Kim, S. Sharif, M. Preobrazhenskaya, J. Schaefer..REDOR constraints on the peptidoglycan lattice architecture of Staphylococcus aureus and its FemA mutant Biochimica et Biophysica Acta (BBA) - Biomembranes (Available online 2 June 2014) DOI:10.1016/j. bbamem.2014.05.
- Printsevskaya SS, Reznikova MI, Korolev AM, Lapa GB, Olsufyeva EN, Preobrazhenskaya MN, Plattner JJ, Zhang YK Synthesis and study of antibacterial activities of antibacterial glycopeptide antibiotics conjugated with benzoxaboroles. Future Med Chem.(2013) Apr;5(6):641-52, ИФ-3,31
- Лапчинская О.А.., Погожева В.В., Пономаренко В.И., Федорова Г.Б., Катруха Г.С., Преображенская М.Н. Штамм Streptomyces griseocarneus subsp. Bleomycini ВКПМ-S887 – продуцент блеомицина А2 и способ получения антибиотика блеомицина А2 Патент на изобретение № 2355758 приоритет от 27 апреля 2007 г
- Sung Joon Kim, Lynette Cegelski, Maria Preobrazhenskaya, and Jacob Schaefer Structures of Staphylococcus aureus cell-wall complexes with vancomycin, eremomycin, and chloroeremomycin derivatives by 13C{19F} and 15N{19F} rotational-echo double resonance Biochemistry. 2006; 45, № 16, 5235-50
- Kirk R. Maples1, Conrad Wheeler, Emily Ip, Jake Plattner, Daniel Chu Maria N. Preobrazhenskaya, Svetlana S. Printsevskaya, Svetlana E. Solovieva, Evgenia N. Olsufyeva, Henry Heine, Julie Lovchik and C. Richard Lyons A Novel Semisynthetic Derivative of Antibiotic Eremomycin Active against Drug-resistant Gram-positive Pathogens Including Bacillus anthracis J. Med.Chem. 2007, # 15, P.p.3681-3685
- Олсуфьева Е.Н., Преображенская М.Н. Изучение cвязи cтруктура-активность в ряду полусинтетических антибиотиков группы полициклических гликопептидов БХ 2006, 32, #4, 339-359 Russian Journal of Bioorganic Chemistry 2006, 32, #4, 303-322 xiaomi mi7 купить
- Jan Balzarini,Els Keyaertsa, Leen Vijgena, Herman Egberink, Erik De Clercq, Marc Van Ransta, Svetlana S. Printsevskaya, Eugenia N. Olsufyeva, Svetlana E. Solovieva, Maria N. Preobrazhenskaya Inhibition Of Feline (Fipv) And Human (Sars) Coronavirus By Semisynthetic Derivatives Of Glycopeptide Antibiotics Antiviral Res 2006, v 72(1), 20-33
- Maria N. Preobrazhenskaya and Eugenia N. Olsufyeva Polycyclic Peptide And Glycopeptide Antibiotics And Their Derivatives As Inhibitors Of Hiv Entry Antiviral Research 2006 v. 71, #2-3, pp 227-236
- S. S. Printsevskaya, S. E. Solovieva, E. N. Olsufyeva, E. P. Mirchink, E. B. Isakova, E. De Clercq, J. Balzarini & M. N. Preobrazhenskaya. Structure-Activity relationships studies on a series of antiviral and antibacterial aglycon derivatives of the glycopeptide antibiotics vancomycin, eremomycin and dechloroeremomycin. J. Med. Chem. 2005. 48, 3885-3890
- Svetlana S. Printsevskaya, Svetlana E. Solovieva#, Evgenia N. Olsufyeva#, Elena P. Mirchink, Elena B. Isakova#, Erik De Clercq, Jan Balzarini & Maria N. Preobrazhenskaya Structure-Activity Relationship Studies of a Series of Antiviral and Antibacterial Aglycon Derivatives of the Glycopeptide Antibiotics Vancomycin, Eremomycin, and Dechloroeremomycin J. Med. Chem.; 2005; 48(11) pp 3885 – 3890)
- S.I. Maffioli, R. Ciabatti, G. Romanò, E. Marzorati, M. Preobrazhenskaya and A. Pavlov Synthesis and antibacterial activity of alkyl derivatives of the glycopeptide antibiotic A40926 and their amides Bioorganic & Medicinal Chemistry Letters Vol 15/16 pp 3801-3805
- Maria N. Preobrazhenskaya, Svetlana S. Printsevskaya, Eugenia N. Olsufyeva, Svetlana Solovieva, Erik De Clercq, Jan Balzarini Derivatives of glycopeptide antibiotics with antiviral activities Drugs of the Future, 2004, 29 (Suppl. A) XVIII th Int. Symp. On MEDICINAL CHEMISTRY, p 375, 2004
- Maria Preobrazhenskaya, Erik De Clercq,Jan Balzarini Glycopeptide antibiotic derivatives Patent filed on Sept. 1, 2003 with application n° PCT/BE03/00144 WO 2004/019970 A2 , August 30, 2002 Publication date 11 March 2004
- Maria N.Preobrazhenskaya† and Eugenia N.Olsufyeva Patents on Glycopeptides of Vancomycin Family and Their Derivatives as Antimicrobials: January 1999 – June 2003* Expert Opinion Ther. Patents (2004) 14 (2):141-173
- Irina S. Severina,Natalya V. Pyatakova,Alexander B. Postnikov,Maria N. Preobrazhenskaya,Yuri V. Khropov Antitumour antibiotic streptonigrin and its derivatives as the inhibitors of nitric oxide-dependent activation of soluble guanylate cyclase European Journal of Pharmacology 483 (2004), 127-132
- M.N.Preobrazhenskaya and Eugenia N.Olsufyeva. Patents on Glycopeptides of Vancomycin Family and Their Derivatives as Antimicrobials: January 1999 - June 2003*. Expert Opinion Ther. Patents (2004) 14 (2):141-173
- S. S. Printsevskaya, A.Y. Pavlov, E. N. Olsufyeva, E. P. Mirchink, and M. N. Preobrazhenskaya. The role of the glycopeptide framework in the antibacterial activity of hydrophobic derivatives of glycopeptide antibiotics. J. Med. Chem. 2003, 46, 1204-1209
- J.Balzarini, Ch. Pannecouque, E. De Clercq,A.Y. Pavlov, S. S. Printsevskaya, O. V. Miroshnikova, M. I. Reznikova, Maria N. Preobrazhenskaya. Antiretroviral Activity of Semisynthetic Derivatives of Glycopeptide Antibiotics. J. Med. Chem., 2003, 46, 2755-2764
- Jan Balzarini, Christophe Pannecouque, Erik De Clercq, Andrey Y. Pavlov, Svetlana S. Printsevskaya, Olga V. Miroshnikova, Marina I. Reznikova, and Maria N. Preobrazhenskaya Antiretroviral Activity of Semisynthetic Derivatives of Glycopeptide Antibiotics J. Med. Chem., 2003, 46 (13), 2755-2764
- Svetlana S. Printsevskaya, Andrey Y. Pavlov, Evgenia N. Olsufyeva, Elena P. Mirchink, and Maria N. Preobrazhenskaya The role of the glycopeptide framework in the antibacterial activity of hydrophobic derivatives of glycopeptide antibiotics J. Med. Chem. 2003, 46 (7) 1204-1209
- S. S. Printsevskaya, A.Y. Pavlov, E. N. Olsufyeva, E. P. Mirchink, E. B. Isakova, M. I. Reznikova, R. C. Goldman, A.A. Branstrom, E.R. Baizman, C. B. Longley, G. Batta and M. N. Preobrazhenskaya. Synthesis and Mode of Action of Hydrophobic Derivatives of the Glycopeptide Antibiotic Eremomycin and De-(N-methyl-D-leucyl)eremomycin Against Glycopeptide-Sensitive and Resistant Bacteria. J. Med. Chem.2002, 45, 1340-1347
- Svetlana S. Printsevskaya Andrey Y. Pavlov, Evgenia N. Olsufyeva, Elena P. Mirchink, Elena B. Isakova, Marina I. Reznikova, Robert C. Goldman, Arthur A. Branstrom, Eugene R. Baizman, Clifford B. Longley, Gyula Batta and Maria N. Preobrazhenskaya Synthesis and Mode of Action of Hydrophobic Derivatives of the Glycopeptide Antibiotic Eremomycin and De-(N-methyl-D-leucyl)eremomycin Against Glycopeptide-Sensitive and Resistant Bacteria J. Med. Chem.2002, 45, # 6, 1340-1347
- Olga V. Leontieva, Maria N. Preobrazhenskaya, and Ralph J. Bernacki Partial circumvention of P-glycoprotein-mediated multidrug resistance by doxorubicin 14-O-hemiadipate Investigational New Drugs, 20, 35-49 (2002)
- Павлов А.Ю., Принцевская С.С., Олcуфьева Е.Н., и Преображенская М.Н. Полусинтетические производные гликопептидного антибиотика эремомицина , активные в отношении гликопептидорезистентных энтерококков Клиническая микробиология и антимикробная химиотерапия № 3, 2001, Приложение 1, стр. 30
- A.Pavlov, O. Miroshnikova, S. Printsevskaya, E. Olsufyeva, M.Preobrazhenskaya, R. Goldman, A. Branstrom, E. Baizman, C. Longley Synthesis of hydrophobic N'-mono- and N',N''-double alkylated eremomycins inhibiting the trans-glycosylation stage of bacterial cell wall biosynthesis J. Antibiotics 54, No 5, 455 – 459 (2001)
- F. Sztaricskai, G. Batta, Z. Dinya, O. Miroshnikova, M. Preobrazhenskaya, F. Hernadi, A. Koncz, Z. Boda Chemical modification of the eremomycin antibiotic. Formation of a cyclic covalent dimer J. Antibiotics 54, No 3, pp. 314 – 319 (2001)
- О.А. Миргородская*, Е.Н.Олсуфьева**, Д.Е.Колуме, Т.Д.Д. Йоргенсен, П. Роепсторфф, А.Ю.Павлов**, О.В.Мирошникова**, М.Н.Преображенская. Изучение димеризации полусинтетических производных эремомицина методом ESI MS и ее роли в проявлении антибактери-альной активности. Биоорг. Хим. 2000, 26, # 8, 631-640
- О.А. Миргородская*, Е.Н.Олсуфьева**, Д.Е.Колуме, Т.Д.Д. Йоргенсен, П. Роепсторфф, А.Ю.Павлов**, О.В.Мирошникова**, М.Н.Преображенская. Изучение димеризации полусинтетических производных эремомицина методом ESI MS и ее роли в проявлении антибактери-альной активности. Биоорг. Хим. 2000, 26, # 8, 631-640
- O.V. Miroshnikova, S. S. Printsevskaya, E. N. Olsufyeva, A.Y. Pavlov, A. Nilius, D. Hensey-Rudloff and M. N.Preobrazhenskayaa Structure-Activity Relationships in the Series of Eremomycin Carboxamides. J. Antibiotics, 2000, v. 53, 286-293
- О.А.Миргородская, Е.Н.Олсуфьева, Д.Е.Колуме, Т.Д.Д. Йоргенсен, П. Роепсторфф, А.Ю.Павлов, О.В.Мирошникова, М.Н.Преображенская Изучение димеризации полусинтетических производных эремомицина методом ESI MS и ее роли в проявлении антибактериальной активности. Биоорг. Хим. 2000, 26, # 8, 631-640
- Olga .Miroshnikova, Svetlana S. Printsevskaya, Eugenia N.Olsufyeva, Andrey Y. Pavlov, Angela Nilius, Dena Hensey-Rudloff and Maria N.Preobrazhenskaya Structure-Activity Relationships in the Series of Eremomycin Carboxamides J. Antibiotics Vol. 53, No 3, 286-293 (2000)
- E. N. Olsufyeva, T. F. Berdnikova, O. V. Miroshnikova, M. I. Reznikova, and M. N. Preobrazhenskaya. Chemical Modification of Antibiotic Eremomycin at the Asparagine Side Chain. J. Antibiotics. 52 (#3), 319-324 (1999).
- А.Ю.ПАВЛОВ, М.Н.ПРЕОБРАЖЕНСКАЯ. Химическая модификация гликопептидных антибиотиков (обзор). Биоорг.хим., 1998, 24, № 9, 644-662.(Russ) A.Y. Pavlov, M.N. Preobrazhenskaya. Chemical modification of glycopeptide antibiotics. Bioorganic Chemistry 1998, vol 24, p.570 - 587 (Engl)
- М.Н.Преображенская,О.В.Мирошникова,А.Ю.Павлов,Е.Н.Олсуфьева Антибактериальные гликопептидные антибиотики (обзор) ХГС 1998, #12, 1605-1631
- M.N.Preobrazhenskaya Second generation drug design among semisynthetic derivatives of glycopeptride antibiotic eremomycin Pharmaceutical Industry Information, vol. 28, No 3, pp 34-40, 1998 (Seoul, South Korea)
- А.Ю.ПАВЛОВ, М.Н.ПРЕОБРАЖЕНСКАЯ Химическая модификация гликопептидных антибиотиков (обзор)Chemical modification of glycopeptide antibiotics Биоорг.хим., 1998, 24, № 9, 644-662 Russian Journal of Bioorganic Chemistry vol 24, No 9, p.570 – 587
- A. Pavlov, M.Preobrazhenskaya, A. Malabarba,R. Ciabatti Synthesis and Antibacterial Activity of Derivatives of the Glycopeptide Antibiotic A-40926 N-alkylated at the Aminoglucuronyl Moiety J. Antibiotics 1998, Vol. 51, pp 525-527
- A. Pavlov, M.Preobrazhenskaya, A. Malabarba,R. Ciabatti Synthesis and Antibacterial Activity of Derivatives of the Glycopeptide Antibiotic A-40926 N-alkylated at the Aminoglucuronyl Moiety J. Antibiotics 1998, Vol. 51, pp 525-527
- Е.Н.Олсуфьева,А.Ю.Павлов,Т.Ф.Бердникова,О.В.Мирошникова,М.Н.Преображенская Поиск антибактериальных препаратов второго поколения в ряду гликопептидных антибиотиков группы ванкомицина Башкирский химический журанал т. б № 4, 41 –45, 1997
- A.Y.Pavlov, E.I.Lazhko and M.N.Preobrazhenskaya. A New Type of Chemical Modification of Glycopeptides Antibiotics: Aminomethylated Derivatives of Eremomycin and Their Antibacterial Activity. J. Antibiotics, Vol. 50, No. 6, 509-513, 1997
- A.Y.Pavlov, E.I.Lazhko and M.N.Preobrazhenskaya A New Type of Chemical Modification of Glyco-peptides Antibiotics: Aminomethylated Derivatives of Eremomycin and Their Antibacterial Activity. J. Antibiotics Vol. 50, No. 6, 509-513, 1997 P. Ferrari, L.Colombo, E.N.Olsufyeva, A.Y.Pavlov, M.I.Reznikova.,E.I.Lazhko Substitution of aminoacids 1 and 3 in teicoplanine aglycon: Synthesis and Antibacterial Activity of three first non-natural dalbaheptides J. Antibiotics, v. 50, # 1, p. 70-81, 1997
- A.Malabarba, R Ciabatti, E.Gerli, R.Ripamonti, P. Ferrari, L.Colombo, E.N.Olsufyeva, A.Y.Pavlov, M.I.Reznikova.,E.I.Lazhko Substitution of aminoacids 1 and 3 in teicoplanine aglycon: Synthesis and Antibacterial Activity of three first non-natural dalbaheptides J. Antibiotics, v. 50, # 1, p. 70-81, 1997
- А.Ю.Павлов,Е.Н.Олсуфьева,О.В.Мирошникова,М.И.Резникова,Э.И.Лажко,А.Малобарба, Р.Чабатти Неприродные агликоны гликопептидных антибиотиков ванкомициновой группы . Синтез и изучение антибактериальной активности Биоорганическая химия 1997. Т.23. N.5. С.849-854
- O.V.Miroshnikova,E.N.Olsufyeva,T.F.Berdnikova,A.Y.Pavlov,M.I.Reznikova,R.Ciabatti,A.Malabarba,E.Colombo. A modification of the N-terminal amino acid in the` eremomycin aglycon J.Antibiotics, v.49, N11,p.1157-1161, 1996
- Omelchuk O.A., Tikhomirov A.S., Shchekotikhin A.E. Annelation of furan rings to arenes. Russ. Chem. Rev. 2016. V.85, №8, Р.817 – 835.
- Tikhomirov A.S., Bykov E.E., Luzikov Y.N., Korolev A.M., Shchekotikhin A.E. Heterocyclic analogs of 5,12-naphtacenequinone 13. Synthesis of 4,11-diaminoanthra[2,3-b]furan-5,10-diones and sulfur-containing analogs. Chem. Heterocycl. Compd. 2016. V.52, №10, Р. 797–802.
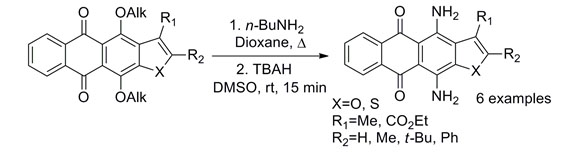
- Shchekotikhin A.E., Dezhenkova L.G., Tsvetkov V.B., Luzikov Y.N., Volodina Y.L., Tatarskiy V.V. Jr., Kalinina A.A., Treshalin M.I., Treshalina H.M., Romanenko V.I., Kaluzhny D.N., Kubbutat M., Schols D., Pommier Y., Shtil A.A., Preobrazhenskaya M.N. Discovery of anti¬tumor anthra[2,3-b]furan-3-carbox¬amides: Optimiza¬tion of synthesis evaluation of anti¬tumor properties. Eur. J. Med. Chem. 2016. V.12., Р.114-129.
- Тевяшова А.Н. Оливомицин А — противоопухолевый антибиотик группы ауреоловой кислоты. Химико-фармацевтический журнал. 2016. Т.50, №7. С.3-8
- Cogoi S.; Zorget S.; Shchekotikhin A.E.; Xodo L.E. Potent apoptotic response induced by chloroacetamidineanthrathiophenediones in bladder cancer cells. Journal of medicinal chemistry. 2015, 58 (14), P. 5476-5485.
- Tikhomirov A.S., Shchekotikhin A.E., Lee Y.-H., Chen Y.-A., Yeh C.-A., Tatarskiy V.V., Dezhenkova L.G., Glazunova V.A., Balzarini J., Shtil A.A., Preobrazhenskaya M.N., Chueh P.J. Synthesis and Characterization of 4,11-Diaminoanthra[2,3 b]furan-5,10-diones: Tumor Cell Apoptosis through tNOX-Modulated NAD+/NADH Ratio and SIRT1. Journal of medicinal chemistry. 2015, 58, 9522−9534.
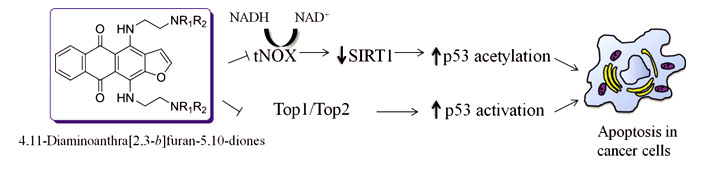
- Lee Y.-R., Chen T.-C., Lee C.-C., Chen C.-L., Ali A., Tikhomirov A., Guh J.-H., Yu D.-S., Huang H.-S.. Ring fusion strategy for synthesis and lead optimization of sulfur-substituted anthra[1,2-c][1,2,5]thiadiazole-6,11-dione derivatives as promising scaffold of antitumor agents Eur. J. Med. Chem., 2015, № 102, P. 661-676.
- Щекотихин А.Е. Противоопухолевые лиганды на основе антратиофендионов: модулирование биологических свойств путем модификации структуры боковых цепей. // Успехи Молекулярной онкологии, 2015, т. 2, вып. 4, стр. 18-19/.
- Shchekotikhin A.E., Glazunova V.A., Dezhenkova L.G., Luzikov Y.N., Buyanov V.N., Treshalina H.M., Lesnaya N.A., Romanenko V.I., Kaluzhny D.N., Balzarini J., Agama K., Pommier Y., Shtil A.A., Preobrazhenskaya M.N. Synthesis and evaluation of new antitumor 3-aminomethyl-4,11-dihydroxynaphtho[2,3-f]indole-5,10-diones. European Journal of Medicinal Chemistry 2014, 30 (86), pp. 797-805.
- Ilyinsky N.S., Shchyolkina A.K., Borisova O.F., Mamaeva O.K., Zvereva M.I., Azhibek D.M., Livshits M.A., Mitkevich V.A., Balzarini J., Sinkevich Y.B., Luzikov Y.N., Dezhenkova L.G., Kolotova E.S., Shtil A.A., Shchekotikhin A.E., Kaluzhny D.N. Novel multi-targeting anthra[2,3-b]thiophene-5,10-diones with guanidine-containing side chains: interaction with telomeric G-quadruplex, inhibition of telomerase and topoisomerase I and cytotoxic properties. European Journal of Medicinal Chemistry 2014, 85 pp. 605-614.
- Moreau P., Dezhenkova L.G., Anizon F., Nauton L., Thery V., Liang S., Kaluzhny D.N., Shtil A.A. New potent and selective inhibitor of pim-1/3 protein kinases sensitizes human colon carcinoma cells to Doxorubicin. Anticancer Agents Medicinal Chemistry 2014, vol. 14 (9) pp. 1228-1236.
- Kovaleva O.A., Tsvetkov V.B., Mamaeva O.K., Ol'shevskaya V.A., Makarenkov A.V., Dezhenkova L.G., Semeikin A.S., Borisova O.F., Shtil A.A., Shchyolkina A.K., Kaluzhny D.N. Preferential DNA photocleavage potency of Zn(II) over Ni(II) derivatives of carboxymethyl tetracationic porphyrin: the role of the mode of binding to DNA. European Biophysics Journal, 2014, vol. 43 (10-11) pp. 545-554.
- Тихомиров А.С., Щекотихин А.Е., Преображенская М.Н. Исследование влияния структуры боковой цепи антра[2,3-b]фуранкарбоксамидов на цитотоксические свойства. Российский Биотерапевтический Журнал, 2014, № 1, с. 133.
- Лесная Н.А., Романенко В.И., Калишьян М.С., Трещалина Е.М., Щекотихин А.Е., Преображенская М.Н. Исследование противоопухолевой активности антрафурандиона ЛХТА-2034 на модели лимфолейкоза Р388/DOX с множественной лекарственной устойчивостью. Российский Биотерапевтический Журнал, 2014, № 1, с. 105.
- Деженкова Л.Г., Цветков В.Б., Штиль А.А. Ингибиторы топоизомераз I и II: химическая структура, механизм действия и роль в химиотерапии опухолей. Успехи Химии, 2014, 83, pp. 82-94.
- Тихомиров А.С., Щекотихин А.Е., Преображенская М.Н. Методы синтеза и модификации линейных антрафурандионов. Химия Гетероциклических Соединений, 2014, № 2, с. 193-208.
- Тихомиров А.С., Щекотихин А.Е., Преображенская М.Н. Методы синтеза и модификации линейных антрафурандионов. Химия Гетероциклических Соединений, 2014, № 2, с. 193-208.
- Тихомиров А.С. Щекотихин А.Е., Лузиков Ю.Н., Королев А.М., Преображенская М.Н. Гетероциклические аналоги 5,12-нафтаценхинона 12. Синтез 2-замещённых производных 4,11-диметокси-5,10-диоксоантра[2,3-b]фуран-3-карбоновых кислот. Химия Гетероциклических Соединений, 2014, № 2, с. 298-308.
- Tikhomirov A.S. Shchekotikhin A. E., Luzikov Yu.N., Korolev A.M., Preobrazhenskaya M. N. Pd-catalyzed cross-coupling/heterocyclization domino reaction: facile access to anthra[2,3-b]furan-5,10-dione scaffold. Tetrahedron, 2014, vol. 70, p. 8062-8066.
- Тевяшова А.Н. Создание пролекарств на основе антрациклиновых антибиотиков. Тонкие химические технологии. 2014. Т. 9. № 6. С. 11-25.
- Тихомиров А.С., Щекотихин А.Е., Деженкова Л.Г., Штиль А.А., Преображенская М.Н. Исследование влияния заместителя в положении 2 на биологические свойства противоопухолевых антрафурандионов Российский биотерапевтический журнал. 2013. Т. 12. № 2. С. 83-86
- Tikhomirov A.S., Shchekotikhin A.E., Luzikov Yu.N., Korolev A.M., Preobrazhenskaya M.N. Heterocyclic analogs of 5,12-naphthacenequinone. 11. A new method for preparing 4,11-dimethoxyanthra[2,3-b]furan-5,10-dione. Chemistry of Heterocyclic Compounds, 2013, 49, P. 241-8, ИФ-0,634
- Cogoi S., Shchekotikhin A.E., Membrino A., Sinkevich Y.B.,Xodo L.E. Guanidino anthrathiophenediones as G-quadruplex binders: Uptake, intracellular localization, and anti-Harvey-ras gene activity in bladder cancer cells. Journal of Medicinal Chemistry 56 (7), pp. 2764-2778
- Королев, M. Н. Преображенская. Гетероциклические аналоги 5,12-нафтаценхинона. 11. Новый метод получения 4,11-диметоксиантра[2,3-b]-фуран-5,10-диона. Химия гетероциклических соединений. 2013, №2, 264—72
- Anna N Tevyashova, Nikita A Durandin, Alexander M Vinogradov, Victor B Zbarsky1,Marina I Reznikova, Lyubov G Dezhenkova, Eugeniy E Bykov, Eugenia N Olsufyeva,Vladimir A Kuzmin, Alexander A Shtil and Maria N Preobrazhenskaya. Role of the acyl groups in carbohydrate chains in cytotoxic properties of olivomycin A.The Journal of Antibiotics (2013), 1–8
- С. Тихомиров, А. Е. Щекотихин, Ю. H. Лузиков, А. М. Королев, M. Н. Преображенская. Гетероциклические аналоги 5,12-нафтаценхинона. 11. Новый метод получения 4,11-диметоксиантра[2,3-b]-фуран-5,10-диона. Химия гетероциклических соединений. 2013, №2, 264—72
- Andrey E. Shchekotikhin,*, Valeria A. Glazunova, Lyubov G. Dezhenkova, Yuri N. Luzikov, Yuri B. Sinkevich, Leonid V. Kovalenko, Vladimir N. Buyanov, Jan Balzarini, Fong-Chun Huang, Jing-Jer Lin, Hsu-Shan Huang, Alexander A. Shtil, Maria N. Preobrazhenskaya. Synthesis and cytotoxic properties of 4,11-bis[(aminoethyl)amino]anthra[2,3-b]thiophene-5,10-diones, novel analogues of antitumor anthracene-9,10-diones. BMC 2009, 17, 1861-1869
- Anna N. Tevyashova a, Eugenia N. Olsufyeva a, Konstantin F. Turchin a, Jan Balzarini b, Eugenyi E. Bykov a, Lyubov G. Dezhenkova a, Alexander A. Shtil c, Maria N. Preobrazhenskaya a,* Reaction of the antitumor antibiotic olivomycin I with aryl diazonium salts. Synthesis, cytotoxic and antiretroviral potency of 5-aryldiazenyl-6-O-deglycosyl derivatives of olivomycin I Bioorganic & Medicinal Chemistry 17 (2009) 4961–4967
- Shchekotikhin A.E., Luzikov Y.N., Buyanov V.N., Preobrazhenskaya M.N. Heterocyclic analogues of 5,12-naphthacenequinone. 8. Synthesis of furanоanthraquinones. Chem. Heterocyclic Compounds, (E ngl. Ed.), 2009, № 2, p. 191-202
- Andrey E. Shchekotikhin, Lyubov G. Dezhenkova, Olga Yu. Susova, Valeria A. Glazunova, Yuri N. Luzikov, Yuri N. Sinkevich, Vladimir N. Buyanov, Alexander A. Shtil, and Maria N. Preobrazhenskaya.. Naphthoindole-based analogues of tryptophan and tryptamine: synthesis and cytotoxic properties. BMC, 2007, 15, 2651-2659
- Andrey E. Shchekotikhin, Valeria A. Glazunova, Yuri N. Luzikov, Vladimir N. Buyanov, Olga Yu. Susova, Alexander A. Shtil and Maria N. Preobrazhenskaya. Synthesis and structure–activity relationship studies of 4,11-diaminonaphtho[2,3-f]indole-5,10-diones BMC 14 (2006) 5241-5251
- A. E. Shchekotikhin, A.A. Shtil, Y. N. Luzikov, T.V. Bobrysheva, V.N. Buyanov and M. N. Preobrazhenskaya. 3-Aminomethyl derivatives of 4,11-dihydroxynaphtho[2,3-f]indole-5,10-dione for circumvention of anticancer drug resistance. Bioorganic & Medicinal Chemistry, 2005, V. 13, P. 2285-2291
- A. N.Tevyashova, A. A. Shtil, E. N. Olsufyeva, V. S. Simonova, A.Samusenko and M. N. Preobrazhenskaya. Carminomycin, 14-Hydroxycarminomycin and Its Novel Carbohydrate Derivatives Potently Kill uman Tumor Cells and Their Multidrug Resistant Variants. J. Antibiotics, 2004, v 57, No2, 143-150
- A. E. Shchekotikhin, V. N. Buyanov, M. N. Preobrazhenskaya. 'Synthesis of 1-(w-alkylamino)naphthoindolediones with antiproliferative properties. Bioorganic & Medicinal Chemistry.2004 Vol 12/14 pp 3923-3930
- E. N. Olsufyeva, A.N. Tevyashova, I. D.Trestchalin, M. N. Preobrazhenskaya, D. Platt, and A. Klyosov . Synthesis and Antitumor Activity of New D-Galactose Containing Derivatives of Doxorubicin. Carb. Res. 338,(13), 1359- 367.(2003)
- Olga V. Leontieva, Maria N. Preobrazhenskaya, and Ralph J. Bernacki. Partial circumvention of P-glycoprotein-mediated multidrug resistance by doxorubicin 14-O-hemiadipate. Investigational New Drugs, 20, 35-49 (2002)
- E.N.Olsufyeva, N.P. Todorova, J.Balzarini, E. DeClercq. Daunorubicin derivatives obtained from daunorubicin and nucleoside dialdehydes. Nucleosides.Nucleotides, v. 16, # 1-2, 87-95, 1997
- Esvan Y.J., Giraud F., Pereira E., Suchaud V., Nauton L., Théry V., Dezhenkova L.G., Kaluzhny D.N., Mazov V.N., Shtil A.A., Anizon F., Moreau P.. Synthesis and biological activity of pyrazole analogues of the staurosporine aglycon K252c. Bioorg. Med. Chem. 2016. V.24, Р.3116-3124
- Alexander Y. Simonov, Evgeny E. Bykov, Sergey A. Lakatosh , Yury N. Luzikov , Alexander M. Korolev, Marina I. Reznikova, and Maria N. Preobrazhenskaya. Macrolactones built on the bis-3,4(indol-1-yl)maleimide scaffold. Tetrahedron, 2014, V. 70, Issue 3, P. 625–630 WOS. 2.772
- С.А.Лакатош, Е.Е.Быков, М.Н.Преображенская CИНТЕЗ 2-ГЕТАРИЛ-3-(ИНДОЛ-1-ИЛ)- И -3-(ПИРРОЛ-1-ИЛ)- МАЛЕИНИМИДОВ И ИЗУЧЕНИЕ ИХ ПРЕВРАЩЕНИЙ ПОД ДЕЙСТВИЕМ ПРОТОННЫХ КИСЛОТ ХИМИЯ ГЕТЕРОЦИКЛИЧЕСКИХ СОЕДИНЕНИЙ. — 2010. — № 10. — С. 1515—1525
- А.Ю. Симонов, С.А. Лакатош, Ю.Н. Лузиков, М.И. Резникова, и М.Н. Преображенская Реакции межмолекулярного и внутримолекулярного нуклеофильного замещения кватернизованных 3-диметиламинометильных производных 3,4-бис (индол-1-ил) малеинимида и 3-(индол-1-ил)-4-(индолин-1-ил) малеинимида ИЗВАН серия химическая 2010, №7, 1409-1417
- А.Ю.Симонов, С.А.Лакатош, Ю.Н.Лузиков, М.и.Резникова, О.Ю.Сусова, А.А.Штиль, С.М.Елизаров, В.Н.Даниленко, М.Н.Преображенская. Синтез 4-замещённых 3-[3-(диалкиламинометил)-индол-1-и]малеинимидов и изучение их способности ингибировать протеинкиназу C-α, предотвращать развитие множественной лекарственной устойчивости опухолевых клеток и цитотоксичности "Известия Академии наук. Серия химическая" 2008, 9, 1-9
- Е.Е.Быков,М.Н.Преображенская.Квантово-химическое изучение циклизации вицинально замещённых гетероциклических аналогов 3,4-бис(индол-1-ил)малеинимидов под действием протонных кислот "Известия Академии наук. Серия химическая" 2008, 7, 1348-1352
- Е.Е.Быков, С.А.Лакатош, М.Н.Преображенская.Квантово-химическое изучение трансформации 2-(N-алкиламино)-3-(индол-1-ил)- и 2-(N-алкиламино)-3-(индол-3-ил)малеинимидов под действием протонных кислот: механизм гидридного переноса с последующей циклизацией "Известия Академии наук. Серия химическая" 2006, 12, 2069-2073
- Е.Е.Быков, С.А.Лакатош, М.Н.Преображенская.Квантово-химическое изучение трансформации 3,4-бис-индолилмалеинимидов с различным сочленением индольных и малеинимидных фрагментов под действием протонных кислот. Известия Академии наук. Серия химическая, 2006, №5,754-760
- S. A. Lakatosh, Y.N. Luzikov and M. N. Preobrazhenskaya. Diazepines[1,4] annelated with indoline and maleimide from 3-(di)alkylamino-4-(indol-1-yl)maleimides: mechanism of rearrangement and cyclization. Tetrahedron, 2005, V. 61, p. 2017-2020
- S. A. Lakatosh, Y. N. Luzikov and M. N. Preobrazhenskaya. Synthesis of 4-substituted 3-(indol-3-yl)maleimides and azepines with annelated indole and maleimide nuclei. Tetrahedron, 2005, v 34, 8241-8248
- S. A. Lakatosh, Y. N. Luzikov, and M. N. Preobrazhenskaya. Synthesis of 6H-pyrrolo[3',4':2,3][1,4]diazepino[6,7,1-hi]indole-8,10(7H,9H)-diones using 3-bromo-4-(indol-1-yl)maleimide scaffold. Organic and Biomolecular Chemistry 2003, 1, 826-833
- Щекотихин. А.Е. Научный путь М. Н. Преображенской. Chem. Heterocycl. Compd. 2016. V.52, №10
- Durandin N.A., Tsvetkov V.B., Bykov E.E., Kaluzhny D.N., Lavrenov S.N., Tevyashova A.N., Preobrazhenskaya M.N. Quantitative parameters of complexes of tris(1-alkylindol-3-yl) methylium salts with serum albumin: Relevance for the design of drug candidates. Journal of Photochemistry & Photobiology, B: Biology. 2016. V.162, P.570–576.
- Е. Е. Быков, Н. Д. Чувылкин, С. Н. Лавренов, М. Н. Преображенская КВАНТОВО-ХИМИЧЕСКОЕ ИЗУЧЕНИЕ ДИССОЦИАЦИИ СОЛЕЙ ТРИИНДОЛИЛМЕТИЛИЯ В СРАВНЕНИИ С ТРИФЕНИЛМЕТИЛХЛОРИДОМ И ЕГО ПРОИЗВОДНЫМИ, ХИМИЯ ГЕТЕРОЦИКЛИЧЕСКИХ СОЕДИНЕНИЙ. — 2011. — № 10. — С. 1485—1490
- Е. В. Степановa,A. A. Штиль,С. Н. Лавренов,В. М. Бухман,А. Н. Иншаков,E. П. Мирчинк,A. С. Тренин,О. А. Галатенко,E. Б. Исакова,В. А. Глазунова,Л. Г. Деженкова,Э. Ш. Соломко,Е. Е. Быков, М. Н. Преображенская Соли трис(1-алкилиндол 3-ил)метилия —новый класс противоопухолевых соединений Известия Академии наук. Серия химическая, 2010, № 12
- Е. Е. Быков, Н. Д. Чувылкин, С. Н. Лавренов, М. Н. Преображенская КВАНТОВО-ХИМИЧЕСКОЕ ИЗУЧЕНИЕ НУКЛЕОФИЛЬНОГО ЗАМЕЩЕНИЯ В ПРОТОНИРОВАННОМ ТРИИНДОЛИЛМЕТАНЕ ХИМИЯ ГЕТЕРОЦИКЛИЧЕСКИХ СОЕДИНЕНИЙ. — 2010. — № 10. — С. 1526—1532
- Sergey N. Lavrenov,Yuriy N. Luzikov,Evgeniy E. Bykov,Marina I. Reznikova,Valeria A. Glazunova,Alexander A. Shtil, and Maria N. Preobrazhenskaya Synthesis and Cytotoxic Potency of Novel Tris(1-alkylindol-3-yl)methylium Salts: Role of N-alkyl Substituents Bioorganic & Medicinal Chemistry 18 (2010) 6905–6913
- Hanh H. Nguyen, Sergey N. Lavrenov, Shyam N. Sundar, David H.H. Nguyen , Min Tseng , Crystal N. Marconett , Jenny Kung, Richard E. Staub b, Maria N. Preobrazhenskaya, Leonard F. Bjeldanes, and Gary L. Firestone, 1-Benzyl-indole-3-carbinol is a novel indole-3-carbinol derivative with significantly enhanced potency of anti-proliferative and anti-estrogenic properties in human breast cancer cells Chemico-Biological Interactions Volume 186, Issue 3, 5 August 2010, Pages 255-266
- А.М.Королев,Ю.Н.Лузиков,М.И. Резникова,М.Н.Преображенская 3-O-и 2-С алкилирование солей L-аскорбиновой кислоты бензилгалогенидами и производными N-замещенных индолметанолов ИЗВАН серия химическая 2010, 201, №2, 447-452
- Е.Е.Быков, С.Н.Лавренов, М.Н.Преображенская Квантовохимическое исследование зависимости pKa от рассчитанной энергии отрыва протона для некоторых производных индола и фенола. Химия гетероциклических соединений, 2006, №1 с.47-50
- S. N. Lavrenov, K.F. Turchin, A.M. Korolev, Olga S. Anisimova and Maria N. Preobrazhenskaya. New pyrano[3,4-b]indoles from 2-hydroxymethyl-indole and L-dehydroascorbic acid. Tetrahedron, 2005, V. 61, 6610-6613
- S.N. Lavrenov, N. P. Solovyeva, M.I. Reznikova, O.S. Anisimova, and M.N. Preobrazhenskaya. The interaction of per-o-acetylated acyclic 1-(1-butylindol-3-yl)-1-deoxy-ketoses with silylated uracil. Nucleosides, Nucleotides & Nucleic Acids Vol. 23, N1&2, pp281-289 (2004)
- A. M. Korolev, A. E. Shchekotikhin, L. N. Lysenkova, and M. N. Preobrazhenskaya. Synthesis of (Indol-3-yl)methanesulfonamide and its 5-Methoxy Derivative. Synthesis 2003, # 3, 383-388.
- S.N. Lavrenov, A.M. Korolev, M.I. Reznikova, A.V. Sosnov and M.N. Preobrazhenskaya. Study of 1-deoxy-1-(indol-3-yl)-L-sorbose, 1-deoxy-1-(indol-3-yl)-L-tagatose and their analogs. Carb. Res. V. 338, # 2, 143-152 (2003)
- S.N. Lavrenov, S. A. Lakatosh, L. N. Lysenkova, A. M. Korolev, M. N. Preobrazhenskaya. Synthesis of Methyl 6-and 5-Nitroindole-2-carboxylates by Nitration of Indoline-2-carboxylates. Synthesis 2002, # 3: 320-322
- Л. Н. Лысенкова, М. И. Резникова, А. М. Королев и М. Н. Преображенская. Изучение превращений 2-с-[(индол-3-ил) метил]- -l-ксило-гекс-3-улофуранозо-новой кислоты (от-крытой формы аскорбигена) в кислой среде. Изв. РАН, серия химическая 2001, № 7, 1248-1252
- S.N. Lavrenov, A.M. Korolev and M.N. Preobrazhenskaya. O-Glycosides of N-hydroxyindoles. Nucleosides, Nucleotides & Nucleic acids 2001, 20, #10-11, 1881-1889
- A. M. Korolev, L. N. Yudina, I. I. Rozhkov, L.N. Lysenkova, E. I. Lazhko, Y. N. Luzikov, and M. N. Preobrazhenskaya. The formation of 2-hydroxy-4-hydroxymethyl-3-(indol-3-yl)cyclopent-2-enone derivatives from ascorbigens. Carbohydrate Research. V.330, # 4, pp 469 - 477. (2001)
- M. I. Reznikova, A. M. Korolev, D. A. Bodyagin and M. N. Preobrazhenskaya. Transformations of ascorbigen in vivo into ascorbigen acid and 1-deoxy-1-(indol-3-yl)ketoses. Food Chemistry. 71(4), 469-474 (2000)
- М. Н Преображенская, А. М Королев. Indole Derivatives in Vegetables of the Cruciferae Family. A review. Russian Journal of Bioorganic Chemistry. 2000, 26, 85-97
- M. Preobrazhenskaya*, I. Rozhkov, E. Lazhko, A. Korolev. Interaction of L-Ascorbic acid with DL-N-methyl-?-hydroxytryptamine. Tetrahedron letters, 39, No 1, 109-112, 1998
- I.I.Rozhkov, E.I.Lazhko,L.N.Yudina, A.M.Korolev Reaction of Vanilmandelic acid and 4-hydroxybenzylalcohol derivatives with L- ascorbic acid Tetrahedron Vol. 53, No. 20, 6971-6976, 1997 Corrigendum: Tetrahedron, 55 (1999) 1517
- Ватлин А.А., Беккер О.Б., Лысенкова Л.Н., Королев А.М., Щекотихин А.Е., Даниленко В.Н. Секвенирование и анализ резистома Streptomyces fradiae ATCC 19609 c целью разработки тест-системы для скрининга новых антибактериальных систем. Генетика. 2016 Т.52, №6, С.723-727.
- Tevyashova A.N., Korolev A.M., Trenin A.S., Dezhenkova L.G., Shtil A.A., Polshakov V.I., Savelyev O.Y., Olsufyeva E.N. New conjugates of polyene macrolide amphotericin B with benzoxaboroles: synthesis and properties. J. Antibiotics. 2016. V.69, P. 549-560.
- Лысенкова Л.Н., Савельев О.Ю., Королев А.М., Даниленко В.Н., Беккер О.Б., Мавлетова Д.А., Ватлин А.А., Омельчук О.А., Щекотихин А.Е.. Синтез 33-(R,S)-бромо-33-дезоксиолигомицина А. Макрогетероциклы. 2016, Т.9, №3, С.307-313.
- Lysenkova L.N., Godovikov I.A., Korolev A.M., Danilenko V.N., Bekker O.B., Mavletova D.A., Vatlin A.A., Shchekotikhin A.E., Preobrazhenskaya M.N. Synthesis and anti-actinomycotic activity of the thiocyanato derivative of oligomycin A modified in the 2-hydroxypropyl side chain. Macroheterocyles, 2015, V.8, № 4, doi: 10.6060/mhc151084s.
- Vatlin A.A., Bekker O.B., Lysenkova L.N., Danilenko V.N. Draft Genome Sequence of Streptomyces fradiae olg1-1, a Strain Resistant to Nitrone-Oligomycin. Genome Announcements, 2015 Sep-Oct; 3(5): e01252-15. doi: 10.1128/genomeA.01252-15.
- • Lysenkova L.N., Godovikov I.A., Korolev A.M., Danilenko V.N., Bekker O.B., Mavletova D.A., Vatlin A.A., Shchekotikhin A.E., Preobrazhenskaya M.N. Synthesis and anti-actinomycotic activity of the thiocyanato derivative of oligomycin A modified in the 2-hydroxypropyl side chain. Macroheterocyles, 2015, V.8, № 4, doi: 10.6060/mhc151084s.
- Королев А.М., Олсуфьева Е.Н., Мирчинк Е.П., Исакова Е.Б. Получение и изучение новых карбоксамидов антибиотиков с 4- или 3-аминометилфенилборной кислотой. //Антибиотики и химиотерапия. 2015, т. 60, №9-10, с. 6-10.
- Деженков А.В., Чешков Д.А., Прохоров И.А., Деженкова Л.Г., Щвец В.И., Кириллова Ю.Г. Региоселективное алкилирование гуаниновых производных при получении мономеров пептидно-нуклеиновых кислот. Известия Академии наук, Серия химическая. 2015, №5, стр. 1100-1106.
- Пожарская Н.А., Иванов И.В., Акчурин И.О., Баберкина Е.П., Щекотихин А.Е. Разработка интерактивных материалов для тестирования обучения студентов органической химии. Успехи в химии и химической технологии, 2014, т. XXVIII, №9, с.99-101.
- Cogoi S., Shchekotikhin A.E., Xodo L.E. HRAS is silenced by two neighboring G-quadruplexesand activated by MAZ, a zinc-finger transcription factor with DNA unfolding property. Nucleic Acids Research, 2014, 42 (13), pp. 8379–8388
- L.N. Lysenkova, K.F. Turchin, A.M. Korolev, V.N Danilenko, O.B Bekker, L.G Dezhenkova, A.A. Shtil, M.N Preobrazhenskaya. Study on retroaldol degradation products of antibiotic oligomycin A. The Journal of Antibiotics (2014) 67, 153–158. (Tokyo). doi:10.1038/ja.2013.92.
- Ol’khovich., Sharapova A.V., Lavrenov S.N., Blokhina S.V., Perlovich G. L. Inclusion complexes of hydroxypropyl-b-cyclodextrin with novel cytotoxic compounds: Solubility and thermodynamic properties. Fluid Phase Equilibria 2014, vol. 384, p. 68–72. (IF = 2.241) WOS
- Lysenkova LN, Turchin KF, Korolev AM,Danilenko VN, Bekker OB, Dezhenkova LG,Shtil AA, Preobrazhenskaya MN. Study on retroaldol degradation products of antibiotic oligomycin A.J Antibiot (Tokyo). doi: 10.1038/ja.2013.92
- Lysenkova LN, Turchin KF, Korolev AM, Dezhenkova LG, Bekker OB, Shtil AA, Danilenko VN, Preobrazhenskaya MN. Synthesis and cytotoxicity of oligomycin A derivatives modified in the side chain. Bioorg Med Chem. (2013)1;21(11):2918-24
- Lapa GB, Bekker OB, Mirchink EP, Danilenko VN, Preobrazhenskaya MN Regioselective acylation of congeners of 3-amino-1H-pyrazolo[3,4-b]quinolines, their activity on bacterial serine/threonine protein kinases and in vitro antibacterial (including antimycobacterial) activity. J Enzyme Inhib Med Chem. 2013 Oct;28(5):1088-93, ИФ-1,495
- J. Schaefer, S.J. Kim, M. Singh, M. Preobrazhenskaya. Staphylococcus aureus Peptidoglycan Stem Packing by Rotational-Echo Double Resonance NMR Spectroscopy. Biochemistry,(2013) v 52, 3651-59
- Lyudmila N Lysenkova, Konstantin F Turchin, Alexander M Korolev, Evgenyi E Bykov, Valery N Danilenko, Olga B Bekker, Alexey S Trenin, Sergei M Elizarov, Lyubov G Dezhenkova, Alexander A Shtil and Maria N Preobrazhenskaya. A novel acyclic oligomycin A derivative formed via retro-aldol rearrangement of oligomycin A. The Journal of Antibiotics (2012) 65, 405–411
- Lyudmila N. Lysenkova1, Konstantin F. Turchin1, Valery N. Danilenko2, Alexander M. Korolev1, and Maria N. Preobrazhenskaya1 The first examples of chemical modification of oligomycin A. The Journal of Antibiotics (2010) 63, 17–22.
- Anna N. Tevyashovaa, Eugenia N. Olsufyevaa, Jan Balzarini, Alexander A. Shtilc, Lyubov G. Dezhenkovac, lLadimir M. Bukhmana, Victor b. Zbarsky, Maria n. Preobrazhenskayaa* Modification of the antibiotic Olivomycin I at the 2’-Keto Group of the Side Chain. Novel Derivatives, Antitumor and Topoisomerase I Poisoning Activity. The Journal of Antibiotics (2009) 62, 37–41
- Maria N. Preobrazhenskaya, Evgenia N. Olsufyeva, Svetlana E. Solovieva, Anna N. Tevyashova,Marina I. Reznikova, Yuryi N. Luzikov, Larisa P. Terekhova, Aleksei S. Trenin, Olga A. Galatenko, Ivan D. Treshalin, Elena P. Mirchink, Vladimir M. Bukhman, Ha#vard Sletta, and Sergey B. Zotchev. Chemical modification and biological evaluation of new semi-synthetic derivatives of S44HP, a genetically engineered anti-fungal polyene macrolide antibiotic, J. Med. Chem., 2009, 52 (1), 189-196
- I. S. Severina, N.V. Pyatakova, A. B. Postnikov, M.N. Preobrazhenskaya, Y. V. Khropov. Antitumour antibiotic streptonigrin and its derivatives as the inhibitors of nitric oxide-dependent activation of soluble guanylate cyclase. European Journal of Pharmacology, 483 (2004), 127-132.
- I. S. Severina, N.V. Pyatakova, A. B. Postnikov, M.N. Preobrazhenskaya, Y. V. Khropov. Antitumour antibiotic streptonigrin and its derivatives as the inhibitors of nitric oxide-dependent activation of soluble guanylate cyclase. European Journal of Pharmacology, 483 (2004), 127-132.
- N.V.Holpne-Kozlova, E.I.Lazhko and M.N. Preobrazhenskaya.Transformation of streptonigrin into streptonigrone and biological evaluation of antibiotic streptonigrin and streptonigrone alkyl ethers. J.Antibiotics, v.45, N 2, p.227-234,1992
- V.V.Tolstikov,J.Balzarini, E.De Clercq and M.N. Preobrazhenskaya. Chemical modification of antibiotic streptonigrin: Synthesis and properties of 2-decarboxy-2-amino- streptonigrin (streptonigrone-imine) J.Antibiotics, 1992, v.45, N 6, p.1002-1007
- V.V.Tolstikov, T.D.Oreshkina, T.V.Osipova, F.Sztarickai, J.Balzarini, E.De Clercq, N.V.Holpne-Kozlova M.N.Preobrazhenskaya. Amides of antibiotic streptonigrin and aminodicarboxylic acids or aminosugars, synthesis and biological evaluation. J.Antibiotics, 1992 v.45,N 6, p.1020-1025
Перейти к "Лаборатория химической трансформации антибиотиков"